| Jun 07, 2013 |
Building an army of one
|
|
(Nanowerk News) Long before humans understood the principles of genes and heritability, they were already shaping animal evolution through selective breeding. The various domesticated species we cultivate today are the product of a centuries-long project of gradual selection and mating to generate strains with optimized physical and behavioral characteristics.
|
|
Nowadays, scientists have amassed considerable insight into animal genetics. Additionally, cloning technology theoretically offers the potential to replicate large numbers of livestock or research animals with identical, desirable traits. In reality, however, most efforts in this area have so far met with disappointment. Now, recent progress made by a research team led by Teruhiko Wakayama at the RIKEN Center for Developmental Biology suggests that multigenerational cloning may soon become a feasible option ("Successful Serial Recloning in the Mouse over Multiple Generations").
|
|
For over a decade, Wakayama’s team has pursued the serial recloning of mice using a technique called somatic cell nuclear transfer (SCNT), which allows generation after generation of identical mice to be produced from a single-source donor. The mouse is an extremely well characterized animal model and should therefore be ideal for testing such strategies. However, Wakayama and his colleagues previously achieved only limited success. “Nobody had succeeded in continuous recloning for more than six generations,” he says.
|
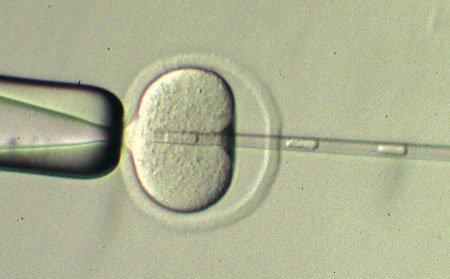 |
| Figure 1: Cloning begins with the injection of the nucleus of a somatic cell from the animal being cloned into an oocyte from which the nucleus has been removed. (© 2013 Teruhiko Wakayama, RIKEN Center for Developmental Biology)
|
|
In general, SCNT is plagued by inefficiency, requiring a substantial investment to successfully obtain healthy cloned animals. Although steady progress in cloning technology has improved the odds, the root causes of failure in the recloning process remain obscure. Despite the difficulties, however, Wakayama was committed to overcoming this challenge. “I am always trying difficult studies where nobody has succeeded previously, and so I wanted to try this again,” he explains.
|
|
Growing an unusual family tree
|
|
SCNT involves extracting the nucleus from an adult donor cell and transferring it into a recipient oocyte from which the nucleus has been removed (Fig. 1). The environment of the oocyte induces a series of ‘reprogramming’ events in the donor nucleus, which subsequently behaves as if it were the nucleus of a newly fertilized egg. If successful, the resulting developmental process yields an embryo that is a clone of the donor animal.
|
|
The reprogramming process requires selective enzymatic addition of chemical modifications to targeted sites on the chromosomes, which in turn modulate expression of nearby genes through ‘epigenetic’ regulation. Certain other enzymes can undermine cloning success by removing these epigenetic marks, prematurely terminating the reprogramming process. Such enzymes can be inhibited by drugs like trichostatin A (TSA), and SCNT success rates can be boosted as much as fivefold using this TSA-facilitated strategy.
|
|
By employing TSA treatment, Wakayama and colleagues have been able to improve their SCNT success rate and achieve a breakthrough in serial recloning. Over the past eight years, the team has repeatedly cloned a single female donor mouse through 25 generations and beyond. From each subsequent generation, the researchers collected the nucleus from one of the ‘cumulus cells’ that surrounds the oocyte, and transferred it into a recipient oocyte. In this manner, they were able to successfully derive a new and healthy clone from every new generation.
|
|
The cloning success rate varied considerably across—and even within—generations: in some cases the rate was as low as 3% and in others as high as 20%. To identify factors that might be altering the rate, the researchers used a variety of control experiments and even accounted for the variability in experimental technique that would inevitably arise across an eight-year experiment. Ultimately, they were unable to identify any direct contributing factors. “There are too many possibilities,” says Wakayama. “Even the season can affect the success rate.”
|
|
Nevertheless, the cloned mice that were generated proved to be healthy, physiologically normal and exhibited typical lifespans. Indeed, a set of clones from generation 20 were each sufficiently fertile to yield normal-sized litters of healthy pups when bred. The animals proved equally healthy at the cellular level, with no evidence of accumulated genetic damage or abnormalities in gene expression relative to first-generation clones. Wakayama compares this achievement to duplicating a DVD versus a videotape. “Copying a videotape will decrease its quality, but copying a digital file does not,” he says. “Thus, cloning by nuclear transfer is more similar to digital than analog copying.”
|
|
A new beginning
|
|
Following the publication of their findings, the team of researchers are continuing to generate clones from each new generation. Wakayama notes that they have reached generation 27 and are collecting data from these animals at present.
|
|
In principle there appears to be no inherent generational limit. As long as TSA is applied to facilitate proper nuclear reprogramming, each round of cloning is no less likely to succeed than the previous round. This suggests that earlier failures in serial recloning were simply the result of an extremely low base success rate, rather than the aggregate effect of cellular defects acquired during each new round of SCNT.
|
|
As TSA and other chemicals with similar inhibitory effects can also dramatically boost the success of SCNT in other species, it is anticipated that serial recloning should prove similarly feasible in larger animals such as cows or pigs. Wakayama also notes that their technique could revive long-lost animal populations. “We plan to use the mouse as a model for resurrecting extinct species,” he says. “For example, we have successfully generated clones from frozen mice, and are trying to make clones from taxidermied animals or from the fur of a mouse.”
|

