| Apr 17, 2014 |
Friction harnessed by proteins helps organize cell division
|
|
(Nanowerk News) A football-shaped structure, known as the mitotic spindle, makes cell division possible for many living things. This piece of cellular architecture, responsible for dividing up genetic material, is in constant flux. The filaments that form it grow and shrink, while motor-like molecules burn energy pushing them about. To ensure the complex process proceeds in an orderly fashion, molecular fasteners pin the filaments together in certain places, and new research in Tarun Kapoor’s Laboratory of Chemistry and Cell Biology helps explain how they do it.
|
|
“These ‘fastener’ proteins do not consume energy, yet they somehow maintain their positions on the filaments, known as microtubules, in spite of all the activity going on,” says Scott Forth, a postdoc in the lab who led the research. “We found these proteins can actually harness this motion to help them do their jobs.”
|
|
In research detailed last week in Cell ("Asymmetric Friction of Nonmotor MAPs Can Lead to Their Directional Motion in Active Microtubule Networks"), the Rockefeller team discovered some of these fastener proteins, known as non-motor microtubule associated proteins, or MAPs, experience different degrees of friction depending on the direction in which they are being moved along a microtubule. As a result, the MAPs can be shuffled into position clustered at the points of the football, for example, without directly consuming any energy.
|
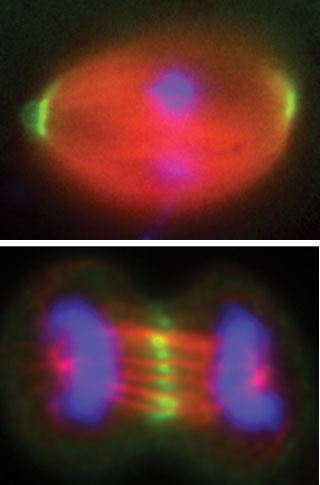 |
| Path of least resistance. Motion of the filaments of the mitotic spindle (red) positions fastener proteins (green) as a cell prepares to divide. One of these proteins, NuMA (top), encounters less resistance as it heads toward the ends of the spindle, while another, PRC1 (bottom), encounters the same amount in either direction, and so remains in the middle.
|
|
By contrast, other proteins involved in this process, known as motor proteins, consume chemical energy to move the microtubules around.
|
|
To figure out how MAPs respond to the movement of the microtubules, the researchers measured the friction generated while each of three MAPs were in contact with moving microtubules. For two of the three, they found an asymmetry: Motion in one direction generated much less friction than motion in the other direction. For example, the NuMA protein experienced less friction when being moved toward the minus end of the microtubule than when being moved toward the plus end, while the EB1 protein showed the opposite, a preference for the plus end.
|
|
Forth compares this asymmetry to a Chinese finger trap. When two fingers inserted into both ends of the trap’s tube are pulled outward, away from one another, the trap tightens, but when both fingers are pushed in together, the trap loosens. “This asymmetry in force is also true for these MAPs. If they are dragged one way along the microtubule it is hard to do it, while if they’re dragged the other way it is easy to do it,” he says.
|
|
Experiments also revealed that as pairs of microtubules were jiggled, these proteins shuffled along them in the direction of least resistance, toward either the plus or minus end of the microtubules. This discovery helps explain how NuMA, for example, stays clustered at the minus ends of the microtubules, where it holds them together and forms a focal point for the microtubules.
|
|
Meanwhile, one of the MAPs, PRC1, showed no asymmetry in friction, a trait that likely keeps it well distributed, rather than clustered, Forth says.
|
|
“Our hypothesis is that maybe this sort of intrinsic mechanical property — the friction these proteins experience — helps them to get where they need to be, as well as maintain the spindle’s organization and mechanical integrity during cell division,” Forth says.
|
|
“Friction plays an important role in our daily lives as we get from one place to another,” says Kapoor, who is the Pels Family Professor. “Similar principles seem to be important for proteins in our cells. In addition to microtubules, there are many biological polymers that are actively moving in cells; for example, DNA while it is being replicated or repaired. It is likely that asymmetries in the friction between proteins and these biopolymers will prove to be important in guiding them to the correct cellular locations.”
|

