| Oct 07, 2016 |
A probe for biofilms unveils bacteria
|
|
(Nanowerk News) The key to overcoming antibacterial resistance could lie in our ability to detect biofilms — a structure formed by bacterial communities that protects them from antibacterial drugs. Scientists at A*STAR have developed the first fluorescent probe to detect biofilms in a living animal model of corneal infection, allowing for access to the bacteria for treatment (Journal of the American Chemical Society, "Detection of Pathogenic Biofilms with Bacterial Amyloid Targeting Fluorescent Probe, CDy11").
|
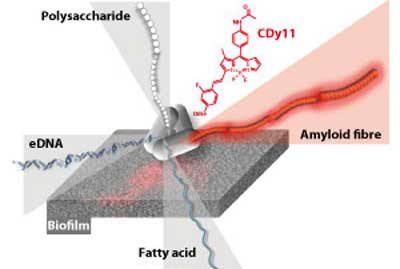 |
| Bacterial biofilms are made of proteins, extracellular DNA (eDNA), polysaccharides and fatty acids. The first biofilm probe, CDy11, developed by A*STAR researchers, binds amyloid, a major protein required for biofilm structure and function. (Image: A*STAR Singapore Bioimaging Consortium)
|
|
Bacterial infections have traditionally been treated using antibiotics. “When bacteria are isolated, they are relatively easy to detect and kill using antibiotics. Once they get together, however, they form a protective structure around their community — a so-called ‘biofilm’,” says Young-Tae Chang, team leader from the A*STAR Singapore Bioimaging Consortium.
|
|
These biofilms — thick substances made of extracellular DNA, proteins, polysaccharides and fatty acids — are a large contributing factor for antibiotic resistance. “It is hard for antibiotics to penetrate the biofilm to reach the bacteria and treat them,” explains Chang.
|
|
To access biofilm-covered bacteria, scientists first need to be able to detect the protective structure. Chang and his team discovered a biofilm probe using a technique previously developed in his lab, called the diversity-oriented fluorescence library approach (DOFLA).
|
|
DOFLA uniquely generates small fluorescent molecules for use as probes by creating simple fluorescent scaffolds that can be modified upon binding to target molecules. DOFL compounds are generated without prior knowledge of a target, which overcomes the limitations of target-oriented approaches, where the applicability of such compounds in complex biological systems is often not guaranteed.
|
|
Chang and his team screened their 10,000-member molecular library for compounds that bind amyloid, a major scaffolding protein in biofilm. They identified the probe, named CDy11 (compound of designation yellow 11), by screening the compounds in high- versus low-amyloid expressing strains of the Pseudomonas aeruginosa bacteria, which has advanced antibiotic resistance mechanisms.
|
|
Chang explains that CDy11 can be used to show where bacteria are hiding and, therefore, which sites to treat. Since amyloid is such a fundamental structure in biofilms, he predicts that CDy11 may have broad applicability. His team has already confirmed that CDy11 can detect biofilms of several strains of bacteria.
|
|
“Currently, there is no direct method to detect or visualize biofilms, so diagnosis is also extremely difficult. Our CDy11 is the first probe to solve the problem,” notes Chang. His team is currently identifying probes to mark other biofilm components and widen the toolbox for biofilm detection.
|

