| Mar 27, 2017 |
Virtual liver model could reduce number of animal experiments
|
|
(Nanowerk News) The liver is crucial for the detoxification of the human body. The exposure to toxins makes it particularly prone to drug-induced injury. Cholestasis, the impairment of bile flow, is therefore a common problem of drug development and occurs for example upon drug overdose. For this reason new active substances must be tested using animal research to prevent liver toxicity.
|
|
Researchers at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden have developed a multi-scale model that can simulate the fluid dynamic properties of bile in the liver (Cell Systems, "A Predictive 3D Multi-Scale Model of Biliary Fluid Dynamics in the Liver Lobule"). The model can help to characterize liver diseases as well as drug-induced injuries.
|
|
The research team is now working on a strategy to calibrate this model to human biliary fluid dynamics. Although animal research will continue to be necessary, the model could contribute to reducing the need for animal research in the future.
|
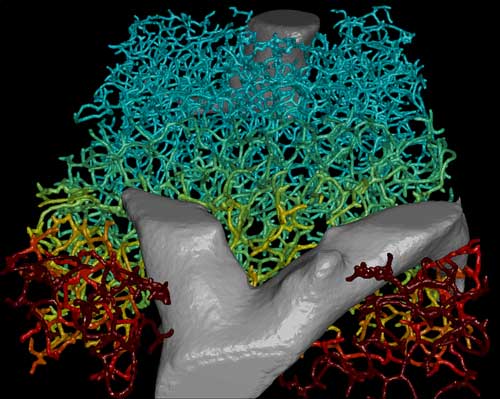 |
| A 3D representation of the geometry of the biliary network with the superimposed bile velocity profile shown as color-code (blue: low velocity; red: high velocity). (Image: MPI f. Molecular Cell Biology and Genetics)
|
|
The liver is the central metabolic organ. In order to digest lipids and excrete waste products, bile is produced in the liver and transported through a highly ramified tubular network to the intestine. A research team headed by Marino Zerial combined high-resolution imaging of the bile canalicular network in the mouse liver with high-resolution 3D analysis of its geometry. They developed a predictive 3D multi-scale model that can simulate the fluid dynamics of bile.
|
|
Although the model currently only represents the network of bile ducts in the mouse liver, the researchers are already working on transposing it to its equivalent in humans.
|
|
“I am certain that we will also be able to apply our model to humans,” says Zerial.
|
|
This means that researchers would not only gain a better understanding of liver diseases but also that they could identify the effects of drugs on the liver.
|
|
“We demonstrated the precision of our model using Paracetamol as an example: The simulation accurately predicted the effects of paracetamol on bile transport.”
|
|
Hence the model is a promising tool for pharmacological drug development – potential side effects of drugs on bile transport could be predicted and tested more precisely.
|
|
Currently, animal research is a legal requirement for the verification of the liver toxicity of new drugs.
|
|
“Animal tests will unfortunately continue to be necessary. But as our method is more sensitive than current toxicology tests we will be able to extract more information from these tests. By this, our model could contribute to reduce the number of animal experiments in drug development in the long term,” explains Zerial.
|

