| Jun 12, 2017 |
Artificial cartilage under tension as strong as natural material
|
|
(Nanowerk News) Biomedical engineers at the University of California, Davis, have created a lab-grown tissue similar to natural cartilage by giving it a bit of a stretch. The tissue, grown under tension but without a supporting scaffold, shows similar mechanical and biochemical properties to natural cartilage. The results are published June 12 in the journal Nature Materials ("Tension stimulation drives tissue formation in scaffold-free systems").
|
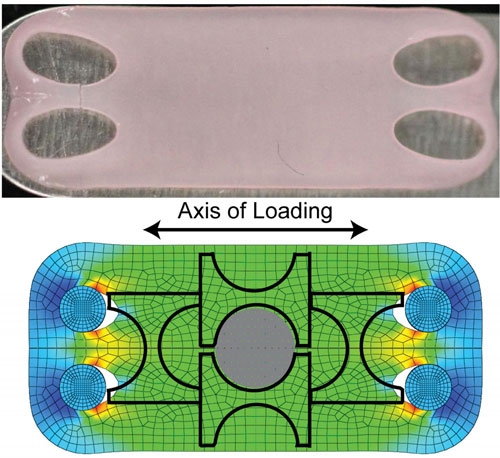 |
| Cartilage neither heals nor regenerates after damage, so artificial cartilage could help many people with joints damaged by wear, injury or disease. Lab-grown cartilage grown with tension (top) shows similar mechanical and chemical properties to natural cartilage, which allows our joints to move smoothly. The lower image shows computer modeling of strain distribution across the artificial tissue. (Image: Athanasiou lab, UC Davis)
|
|
Articular cartilage provides a smooth surface for our joints to move, but it can be damaged by trauma, disease or overuse. Once damaged, it does not regrow and is difficult to replace. Artificial cartilage that could be implanted into damaged joints would have great potential to help people regain mobility.
|
|
Natural cartilage is formed by cells called chondrocytes that stick together and produce a matrix of proteins and other molecules that solidifies into cartilage. Bioengineers have tried to create cartilage, and other materials, in the lab by growing cells on artificial scaffolds. More recently, they have turned to "scaffold-free" systems that better represent natural conditions.
|
|
The UC Davis team, led by Professor Kyriacos Athanasiou, Department of Biomedical Engineering, grew human chondrocytes in a scaffold-free system, allowing the cells to self-assemble and stick together inside a specially designed device. Once the cells had assembled, they were put under tension -- mildly stretched -- over several days. They showed similar results using bovine cells as well.
|
|
"As they were stretched, they became stiffer," said Jerry Hu, a research engineer and co-author on the study. "We think of cartilage as being strong in compression, but putting it under tension has dramatic effects."
|
|
The new material had a similar composition and mechanical properties to natural cartilage, they found. It contains a mix of glycoproteins and collagen, with crosslinks between collagen strands giving strength to the material.
|
|
Experiments with mice show that the lab-grown material can survive in a physiological environment. The next step, Hu said, is to put the lab-grown cartilage into a load-bearing joint, to see if it remains durable under stress.
|
|
"In this comprehensive study, we showed that we can finally engineer tissue that has the tensile and compressive characteristics of native tissue," Athanasiou said. "The artificial cartilage that we engineer is fully biological with a structure akin to real cartilage. Most importantly, we believe that we have solved the complex problem of making tissues in the laboratory that are strong and stiff enough to take the extremely high loads encountered in joints such as the knee and hip."
|

