| Jun 20, 2013 |
Novel cellulose structure requires fewer enzymes to process biomass to fuel
|
|
(Nanowerk News) Improved methods for breaking down cellulose nanofibers are central to cost-effective biofuel production and the subject of new research from Los Alamos National Laboratory (LANL) and the Great Lakes Bioenergy Research Center (GLBRC). Scientists are investigating the unique properties of crystalline cellulose nanofibers to develop novel chemical pretreatments and designer enzymes for biofuel production from cellulosic—or non-food—plant derived biomass.
|
|
“Cellulose is laid out in plant cell walls as crystalline nanofibers, like steel reinforcements embedded in concrete columns,” says GLBRC’s Shishir Chundawat. “The key to cheaper biofuel production is to unravel these tightly packed nanofibers more efficiently into soluble sugars using fewer enzymes.”
|
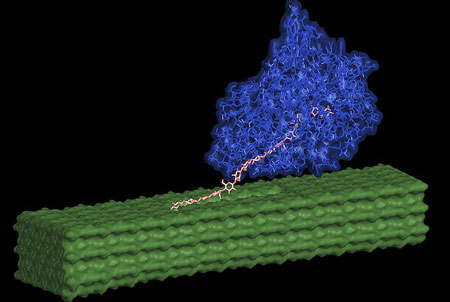 |
| An enzyme (shown in blue) pulls out individual cellulose chains (pink) from the pretreated nanofiber surface (green) and then breaks them apart into simple sugars. (Image: Shishir Chundawat, Great Lakes Bioenergy Research Center)
|
|
An article published this week in the Proceedings of the National Academy of Sciences ("Increased enzyme binding to substrate is not necessary for more efficient cellulose hydrolysis") suggests—counter-intuitively—that increased binding of enzymes to cellulose polymers doesn’t always lead to faster breakdown into simple sugars. In fact, Chundawat’s research team found that using novel biomass pretreatments to convert cellulose to a unique crystalline structure called cellulose III reduced native enzyme binding while increasing sugar yields by as much as five times.
|
|
“The ability of this unconventional pretreatment strategy, currently under development at GLBRC, to selectively alter the cellulose crystal structure may lead to an order of magnitude reduction in enzyme usage. This will be critical for cost-effective cellulosic biofuel production,” says Bruce Dale of Michigan State University, who leads GLBRC’s biomass deconstruction research area.
|
|
The researchers had previously demonstrated that altering the crystal structure of native cellulose to cellulose III accelerates enzymatic deconstruction; however, the recent observation that cellulose III increased sugar yields with reduced levels of bound enzyme was unexpected. To explain this finding, Chundawat and a team of LANL researchers led by Gnana Gnanakaran and Anurag Sethi developed a mechanistic kinetic model indicating that the relationship between enzyme affinity for cellulose and catalytic efficiency is more complex than previously thought.
|
|
Cellulose III was found to have a less sticky surface that makes it harder for native enzymes to get stuck non-productively on it, unlike untreated cellulose surfaces. The model further predicts that the enhanced enzyme activity, despite reduced binding, is due to the relative ease with which enzymes are able to pull out individual cellulose III chains from the pretreated nanofiber surface and then break them apart into simple sugars.
|
|
“These findings are exciting because they may catalyze future development of novel engineered enzymes that are further tailored for conversion of cellulose III rich pretreated biomass to cheaper fuels and other useful compounds that are currently derived from non-renewable fossil fuels,” says Gnanakaran.
|

