| Jan 08, 2014 |
Climate change: How does soil store CO2?
|
|
(Nanowerk News) Global carbon dioxide (CO2) emissions continue to rise – in 2012 alone, 35.7 billion tons of this greenhouse gas entered the atmosphere. Some of this CO2 is absorbed by the oceans, plants and soil. As such, they provide a significant reservoir of carbon, stemming the release of CO2. Scientists have now discovered how organic carbon is stored in soil. Basically, the carbon only binds to certain soil structures. This means that soil’s capacity to absorb CO2 needs to be re-assessed and incorporated into today’s climate models.
|
|
Previous studies have established that carbon binds to tiny mineral particles. In this latest study, published in Nature Communications ("Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils"), researchers have shown that the surface of the minerals plays just as important a role as their size.
|
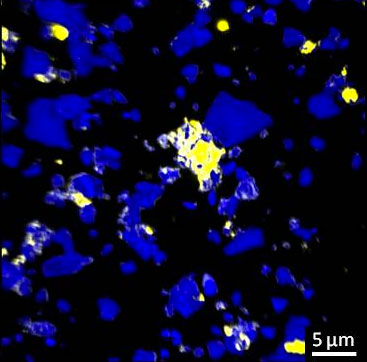 |
| Distribution of organic matter in soil: carbon tends to bind to specific rough mineral surfaces, known as hot spots (yellow areas). (Image: C. Vogel/TUM)
|
|
“The carbon binds to minerals that are just a few thousandths of a millimeter in size – and it accumulates there almost exclusively on rough and angular surfaces,” explains Prof. Ingrid Kögel-Knabner, TUM Chair of Soil Science.
|
|
The role of microorganisms in sequestering carbon
|
|
It is presumed that the rough mineral surfaces provide an attractive habitat for microbes. These convert the carbon and play a part in binding it to minerals. “We discovered veritable hot spots with a high proportion of carbon in the soil,” relates Cordula Vogel, the lead author of the study. “Furthermore, new carbon binds to areas which already have a high carbon content.”
|
|
These carbon hot spots are, however, only found on around 20 percent of the mineral surfaces. It was previously assumed that carbon is evenly distributed in the soil. “Thanks to our study, we can now pin-point the soil that is especially good for sequestering CO2,” continues Kögel-Knabner. “The next step is to include these findings in carbon cycle models.”
|
|
Mass spectrometer helps to visualize molecules
|
|
The sample material used by the team was loess, a fertile agricultural soil found in all parts of the world – which makes it a very important carbon store. The researchers were able to take ultra-precise measurements using the NanoSIMS mass spectrometer. This procedure allowed them to view and compare even the most minute soil structures.
|


