| Sep 22, 2015 |
Sodium-ion batteries are potential power technology of future
|
|
(Nanowerk News) The high cost and scarcity of lithium are driving research to develop alternatives to lithium-ion batteries, especially to meet future needs in energy storage, say researchers from Purdue University in an article about a potential replacement.
|
|
Sodium-ion batteries represent a possible alternative, in large part because of sodium's low cost and natural abundance. However, critical advances are needed for sodium-ion technology to fulfill its promise, said Vilas Pol, an associate professor of chemical engineering.
|
|
The article appeared earlier this month in Current Opinion in Chemical Engineering ("Advancement in sodium-ion rechargeable batteries"). The article was authored by Pol and doctoral students Arthur Dysart and Jialiang Tang.
|
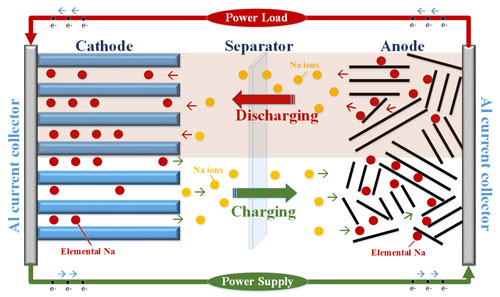 |
| This schematic illustrates the functioning of sodium-ion batteries. The high cost and scarcity of lithium are driving research to develop alternatives to lithium-ion batteries, especially to meet future needs in energy storage. One potential alternative is sodium-ion batteries. (Purdue University image/Vilas Pol) (click on image to enlarge)
|
|
Lithium-ion batteries are used widely in products from consumer electronics to electric vehicles. However, the need for alternatives is being driven by new and expanding applications including batteries to store power from sources such as solar and wind energy for use on the power grid.
|
|
"If everybody wants to start using lithium-ion batteries for multiple purposes, we don't have enough lithium on the planet to sustain that, so we have to find alternatives," Pol said.
|
|
Researchers are working to replace lithium-ion batteries' standard internal components with functioning sodium counterparts.
|
|
Batteries have two electrodes, called an anode and a cathode. The anodes in most of today's lithium-ion batteries are made of thin layers of stacked graphene called graphite. In lithium-ion batteries, lithium ions can easily fit between graphene layers due to their small size. In sodium-ion batteries, sodium ions cannot pass between the graphitic layers reversibly due to their larger size and bulkier nature. The sodium ions go inside the graphitic layers during charging but do not come back during discharging process.
|
|
On the cathode side, new materials have to be developed to replace lithium-containing materials. One option is to replace lithium cobalt oxide cathodes with sodium cobalt oxide cathodes.
|
|
One drawback to sodium-ion batteries is that they are slightly heavier than lithium-ion batteries. However, their low cost and abundant nature compared to lithium-ion batteries may outweigh this concern.
|
|
"The main advantage of sodium-ion batteries is the potential financial benefit," Dysart said. "Lithium is a very scarce element in the world. We could actually experience a lithium shortage in coming years."
|
|
Pol added: "Sodium is more abundant and a thousand times cheaper than lithium. It's even in seawater."
|
|
He is leading research at Purdue to improve sodium-ion batteries by using a variety of tailored carbons and their combinations with sodium-alloying materials such as tin and antimony to potentially double the capacity of the anodes, making possible smaller anodes and reducing the size and weight of the batteries.
|
|
The challenge with sodium-alloying materials is the great volumetric expansion effect once sodium ions form a high-energy chemical bond.
|
|
"It will expand more than 300 percent, and then it shrinks when the battery is discharged as the sodium is taken out," said Pol, also an associate professor of materials engineering. "This great degree of expansion and contraction will cause the anode to fail over time, so we are working on alternative materials breathing architectures to mitigate the expansion."
|
|
Tang said, "Perhaps we can confine the expansion locally, similar to breathing lungs. When we breathe our lungs expand and contract quite a bit, but we don't expand."
|
|
The sodium alloying materials in combination with carbon could bring higher performance anodes that overcome limitations in conventional carbon anodes.
|

