| Jan 25, 2016 |
Sophisticated enzyme-mimic enables efficient hydrogen production
|
|
(Nanowerk News) Researchers from the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences (HIMS) have developed a new catalyst that paves the way for low-cost, large-scale hydrogen-production. The researchers, led by Professor Joost Reek, recently presented their enzyme-mimicking catalyst in Sciences Advances ("An iron-iron hydrogenase mimic with appended electron reservoir for efficient proton reduction in aqueous media").
|
|
A hydrogen-based sustainable economy depends on technology to generate molecular hydrogen using sustainable solar or wind energy. Catalytic chemistry already enables this by using water as feedstock and platinum and iridium as active materials. However, because such elements are scarce and expensive this technology cannot be applied at the required global scale.
|
 |
|
Looking at nature
|
|
To overcome this hurdle, chemists have started looking toward nature where so-called iron-iron hydrogenase enzymes catalyse the conversion (reduction) of protons to molecular hydrogen with a performance comparable to platinum. It is a major challenge to apply these enzymes directly in hydrogen-producing devices since their isolation is difficult and their performance in air poor. Nevertheless, their high efficiency has spurred the search for comparable iron-based molecular complexes that also can produce hydrogen with a platinum-like performance.
|
|
Over the last decade, numerous synthetic complexes have been prepared that structurally and functionally mimic the active site of hydrogenase enzymes. Most of these 'synthetic hydrogenases' show serious drawbacks such as a low efficiency and stability and the requirement of organic solvents. Hydrogenase-mimics that perform efficiently in an aqueous environment while being tolerant to air have until now not been reported.
|
|
Characteristic feature
|
|
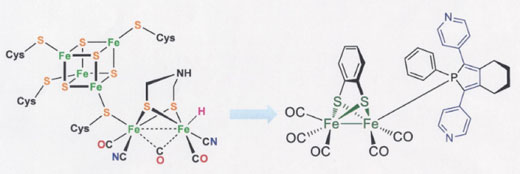
In the current edition of Science Advances, the researchers offer a possible solution by presenting a new oxygen-tolerant iron-iron hydrogenase-mimic that not only achieves efficient proton reduction in aqueous media but also displays electron storage and pre-organisation in close proximity to the active site - a feature characteristic of working natural enzymes.
|
|
The Amsterdam-designed synthetic hydrogenase contains a redox-active phosphorous ligand that acts as an electron reservoir and actively partakes in the reduction of protons. It donates an electron to the active site during the catalytic cycle when needed. As a result, the catalyst displays high turnover numbers (TON) and turnover frequencies (TOF).
|
|
Combining this high performance with the operation in aqueous media and the tolerance towards oxygen, this new hydrogenase-mimic is a major step toward the development of catalysts for inexpensive large-scale hydrogen-producing devices. Further development will be directed toward analogs that operate at lower overpotentials and can be efficiently implemented in devices (for example, by anchoring to electrodes or metal-organic frameworks).
|

