| Jul 04, 2012 |
Nanomaterials: Formation in a flash
|
|
(Nanowerk News) Titanium dioxide, or titania, is an inorganic material commonly used as a whitening agent in food and toothpaste. It is also used as one of the main active ingredients in sunscreens. The properties that make titania useful in commercial applications — namely its whitening ability and high refractive index — are now being exploited in a wide range of technological applications.
|
|
One particular area of interest has been the application of titania in dye-sensitized solar cells — devices that can be used to convert sunlight into electricity. Such application often requires the formation of intricate surface patterns, with the key limiting factors for development being cost and speed of processing. Now, Ramakrishnan Ganesan, Mohammad Saifullah and co-workers at the A*STAR Institute of Materials Research and Engineering have described the use of a technique called step-and-flash imprint lithography (SFIL) to produce such patterns on the nanoscale (see paper in ACS Nano: "Direct Patterning of TiO2 Using Step-and-Flash Imprint Lithography").
|
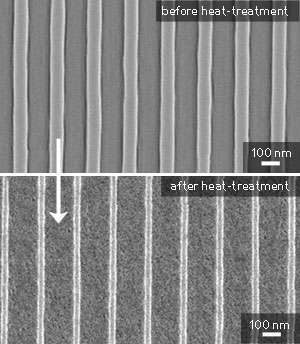 |
| The nanoscale titania pattern before and after heat-treatment. (© ACS)
|
|
“The precursor method to SFIL is thermal nanoimprint lithography, which is extremely time-consuming as it requires temperature-cycling processes to form a pattern,” explains Saifullah. “A mold could be pressed into a heated (and softened) resist material or a liquid precursor could be forced into a mold and then hardened upon heating.”
|
|
Newer processes eliminate the need for heating by using irradiation with ultraviolet (UV) light to harden the polymer. Although this process may be ideal for organic polymer materials, it is more problematic when using inorganic materials such as titania as the liquid precursor materials are highly viscous and do not spread easily. As a result, the dispensing nozzle may sometimes become blocked.
|
|
The chemicals used to make titania can also be unstable in solution, so the team had to identify a mixture of components that offered a combination of stability and low viscosity. “We found that an allyl functionalized titanium complex was stable in combination with other polymer precursors,” explains Saifullah. The final component of the mixture is a photoinitiator — which starts the polymerization process upon irradiation with UV light.
|
|
The mixture was dispensed onto the surface in the form of droplets, and the mold pressed into place to help the liquid spread. Irradiation with UV light results in hardening of the pattern, after which the mold can be removed. A final heating step burns away the organic material, leaving behind a shrunken version of the original pattern made from titania (see image). Significantly, the aspect ratio of the pattern is maintained after the heat-treatment process.
|
|
“Our current method is quite specific to titania, but after tackling this most important material, we hope to develop similar procedures for other inorganic materials,” says Saifullah.
|

