| Aug 24, 2012 |
Efficient catalysis on chiral surfaces
|
|
(Nanowerk News) A team of chemists from ETH Zurich headed by Professor Alfons Baiker has found answers to the question as to why a particular kind of catalysis only really generates one form of a chiral substance. This kind of catalysis is thus becoming increasingly interesting for industry.
|
|
Practice is when everything works but nobody knows why. This light-hearted saying can equally be applied to chemical processes. “The process of so-called heterogeneous asymmetric catalysis is very easy to use in itself and works extremely well,” says Alfons Baiker, an emeritus professor of reaction engineering and catalysis. “Understanding how it works, however, poses a major challenge for research.” In a paper just published in the journal Angewandte Chemie ("Platinum-Catalyzed Asymmetric Hydrogenation: Spectroscopic Evidence for an O-H-O Hydrogen-Bond Interaction between Substrate and Modifier"), Baiker and his team describe a mechanism that is responsible for converting a non-chiral substrate with over 90% yield into the desired of two possible chiral products, a precursor of vitamin B5.
|
 |
| Model of the reaction steps on the chirally modified platinum surface. (Illustration: Prof. A. Baiker’s group / ETH Zurich)
|
|
Chirality as a challenge
|
|
Chiral molecules exist in two mirror forms, so-called enantiomers. They have the same chemical composition but behave like the right hand to the left: laid on top of one another, they cannot be aligned. Because the two enantiomers differ in terms of their biological effect, it is crucial that only one of two possible forms is produced in the production of fine chemicals, pharmaceuticals, flavours and fragrances, or even fertilisers, for instance. Obtaining a high level of enantiopurity in a chemical reaction, however, is a challenge. One way of achieving this is to use certain catalysts, which create a particular reaction centre to determine the chirality (handedness) of a product in a controlled fashion. Experts refer to this as “asymmetric catalysis”.
|
|
Special catalytic process necessary
|
|
Alfons Baiker’s team worked with a special form of this catalytic process, so-called heterogeneous asymmetric catalysis, and analysed it in detail. In the process, a catalytically active noble metal – in this case, platinum – is modified with a suitable chiral molecule in such a way that its surface also becomes handed. The noble metal itself is achiral. The modifier binds the substrate available in solution in such a way that almost only one of the two enantiomers is formed during the reaction.
|
|
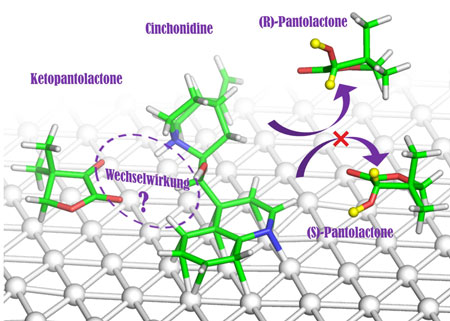
In the case at hand, the modifier is cinchonidine, which adheres to the platinum surface. The substrate , ketopantolactone, binds to the modifier in a highly specific way and picks up hydrogen atoms from the surface of the metal to form (R)-pantolactone, a precursor of vitamin B5 frequently used in industry.
|
|
Years ago, Alfons Baiker and his team already discovered that the modifier and substrate bind to each other via a hydrogen bridge between a nitrogen and an oxygen atom. The chemists thus assumed that only this one bond was needed for a successful reaction, but were unable to explain the well-functioning, “high-percentage” conversion of ketopantolactone into (R)-pantolactone sufficiently.
|
|
New interaction as pointer on the scales
|
|
Using special, complex analysis methods which they devised themselves, the chemists from ETH Zurich have now succeeded in identifying another key interaction between the modifier and substrate. In doing so, the researchers investigated the possible structure of the modifier-substrate complex in detail and came across a previously unknown interaction between the two substances: a hydrogen bond that forms a bridge between two oxygen atoms of the two molecules at a particular point. “This interaction was previously unknown and has a significant effect on the controlled formation of the desired enantiomer,” says Baiker.
|
|
The modifier molecules that adhere to the platinum surface also make a major contribution. They have a movable “head section”, which facilitates the formation of the crucial bonds with the reactive molecule, as the research team was able to prove in earlier studies with the aid of scanning tunnelling microscopy. If there are too many of the bulky modifiers on the surface of the metal, they no longer occupy their preferential position, which means that not only the desired enantiomer is produced anymore.
|
|
Producing tailored modifiers
|
|
Alfons Baiker and his team have been conducting research on heterogeneous catalysis for over twenty years. For him, his latest paper is “merely” one of over 220 publications on this kind of catalysis that have sprung from his group over the years.
|
|
But why do the researchers want to know which mechanism and bonding types underlie this catalysis? “Apart from anything else, so we can produce ‘tailored’ modifiers for the chiral modification of active metal surfaces,” says Baiker. “The structure and bonding types of the modifier and substrate need to match in order to be able to perform asymmetric catalysis.”
|
|
Heterogeneous catalysis with plus points
|
|
To this day, the chemical industry tends to use asymmetric heterogeneous catalysis rarely. It primarily deploys homogeneous catalysis, in which the catalyst and substrate are present in the liquid phase. According to Baiker, however, heterogeneous asymmetric catalysis has several tangible technical advantages, such as the easier separation, regeneration and recycling of the catalyst. Moreover, it enables production processes to be conducted more easily. This, along with the basic knowledge of chiral surfaces gained, is the main driver of his catalysis research, says the emeritus professor.
|
|
“If we succeed in modifying the surfaces of metals specifically for a particular reaction with chiral molecules, it could be extremely important for the production of enantiopure compounds,” says Baiker. The possibility of switching from the production of one enantiomer to the other in a continuous production process by substituting the modifier, for instance, is extremely interesting. This would enable products of a different chirality to be produced in a single operation.
|

