| Nov 07, 2012 |
Coming soon: Tabletop molecular movies
|
|
(Nanowerk News) One of the most urgently sought-after goals in modern science is the ability to observe the detailed dynamics of chemical reactions as they happen – that is, on the spatial scale of molecules, atoms, and electrons, and on the time scale of picoseconds or even shorter.
|
|
That is a formidable challenge. But as a successful 2010 proposal for an ambitious NIST project explains, “it is critical to the development of next-generation nanomaterials ranging from industrial catalysts to renewable energy devices that harvest sunlight, store electricity, and make hydrogen and other fuels from splitting water or recycling carbon dioxide.”
|
|
Now a group of researchers from that ongoing project has devised and demonstrated a highly unusual, compact, and relatively inexpensive x-ray source for an imaging system that may soon be employed to produce the kind of “molecular movies” that scientists and engineers need.
|
 |
| Close-up of water-jet target (vertical line, ~ 0.2 mm wide) used to produce picosecond x-ray pulses. (Credit: Jens Uhlig)
|
|
“I believe that we are going to be able to measure interatomic distances to sub-angstrom accuracy [1 angstrom = 10-10 m],” says Joel Ullom of the Quantum Devices Group in the Physical Measurement Laboratory (PML)’s Quantum Electronics and Photonics Division, Principal Investigator for the collaborative project and head of the team that created the x-ray source. “And we will be able to watch atomic-scale activity with picosecond resolution during chemical reactions.”
|
|
“Joel's x-ray source is a novel table-top system that creates picosecond pulses of x-rays, a holy grail among scientists who are trying to elucidate the precise, real-time motion of electrons, atoms, and molecules,” says Marla Dowell, leader of PML’s Sources and Detectors Group. “Eventually, this table-top approach will be able to compete head-to-head with far more expensive and elaborate synchrotron techniques.”
|
|
The operating principle is extraordinary. It begins with a pulsed infrared (IR) laser beam, which is split into two parts. The first part is used to photoexcite a material under study, starting a chemical reaction. The second part is routed into a vacuum chamber, above which is a water reservoir that has a tiny aperture leading to the chamber. Water is drawn into the chamber in a 0.2 mm wide jet (see figure, above right) and the laser beam is focused onto the streaming water jet target.
|
|
“This ignites a plasma on the target,” Ullom says, “and some of the electrons from the ionization are accelerated – due to the very large electric fields from the laser – back into the water target. There they undergo the same kind of abrupt deceleration that electrons do in a conventional x-ray tube. The IR beam has very little energy per photon. But what comes out of the interaction with the target are x-rays with energies 10,000 times higher. Then we collimate the x-ray beam so that it strikes the sample of interest.” The x-rays then pass through the sample and into a separate cryogenic chamber where superconducting x-ray detectors record the absorption spectrum. (See figure at bottom of page.)
|
|
“So the system contains two strikingly different environments: detectors at millikelvin temperatures, and a plasma source with surface-of-the-sun temperatures,” Ullom says. “And they are only about 15 cm apart. We address that by placing many layers of IR blocking filters in the detector enclosure. They transmit x-rays but stop visible and IR radiation.”
|
|
The source’s x-ray output is broadband, with photon energies ranging from a few hundred electron volts (eV) to roughly 15 or 20 keV. That range is important, Ullom says, because different x-ray energies are absorbed by different elements and different combinations of orbital configurations and interatomic spacings: “The ability of a material to absorb x-rays depends very sensitively on the chemical state of the atom, and the signal is markedly affected at ‘edges’ between different sets of orbital transitions. In addition, ripples in the absorption spectrum, caused by interference patterns in the wave function of electrons, reveal an atom’s distance to its nearest neighbor.”
|
|
In September, the team demonstrated that the x-ray source was stable over substantial time intervals. The next step is to begin doing science with it. “We’re very interested in photoactive materials, components for next-generation solar cells and catalysts,” Ullom says. “We’ll start with model systems and go from there.
|
|
“There are some materials which, when integrated into a sensitized solar cell, are known to produce better performance by the kinds of bulk metrics that matter most in practical terms, such as the efficiency of energy creation. But it’s not always clear why one particular material is better than another. So if we can see an electron moving from, say, one part of a molecule to another, then that can help explain a particular material’s advantage.”
|
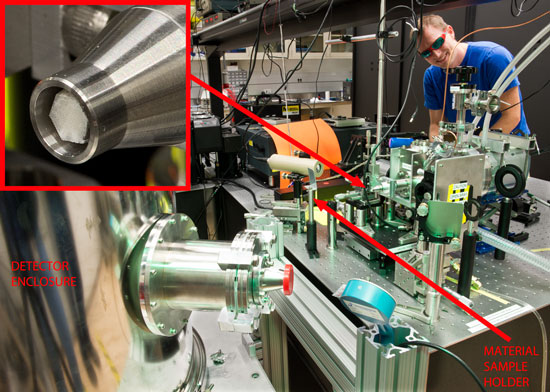 |
| Postdoctoral researcher Galen O'Neil stands behind tabletop apparatus. Inset image at top left is an enlarged view of the polycapillary optic (a collection of very fine glass tubes) used to capture and refocus x-rays from the plasma source onto the sample.
|
|
Of course, photovoltaic systems are not designed to respond to respond to x-ray frequencies. So the sample is excited, or “pumped,” by the other half of the original IR beam. “That beam illuminates the target at wavelengths relevant to its behavior,” Ullom says, “and then the x-ray beam hits it. The two pulses are very precisely synchronized, so we can control the delay between them. We can start a photoreaction with the first half of the pulse, wait a known delay time, say 50 ps, and then bring in the x-rays to give us a snaphot of the reaction at that point. Then we can change the delay interval to observe other stages of the reaction.”
|
|
The chief challenge to date has been making the water jet operation work continuously and dependably, without freezing solid in the vacuum chamber. “Water is a somewhat counter-intuitive choice for a source,” Ullom says. “You get more x-rays if you hit something heavier, like copper, and other people working on related designs have typically used metal wires moving from one spool to another so that the ionizing beam never hits the same place twice.
|
|
“We did not want to do that for a variety of reasons. One is that spallation will coat your optics. Water might land on our optics, but it’s immediately pumped away, and we don’t have to worry about inhaling metal particulates. Also, when electrons hit a dense material, you get both broadband output and sharp emission lines. But for absorption spectroscopy, those lines are actually an inconvenience. Water has no such lines.”
|
|
The pulsed x-ray program -- whose team consists of condensed matter physicists, physical chemists, and laser experts, as well as information-processing experts from NIST and academic institutions -- will begin operation with a detector array of 160 elements, with plans to expand to 1000 elements during the remaining three years of the initial project funding.
|
|
Expectations are high. The NIST project, Dowell says, “has the potential to open the field of time-resolved X-ray imaging to a much broader scientific community, similar to the way Carl Wieman introduced the semiconductor diode laser to the atomic physics community.”
|


