| Feb 19, 2013 |
For the first time, scientists see how TiO2 surface defects halt photodecomposition
|
|
(Nanowerk News) Using chemical imaging techniques, scientists at Pacific Northwest National Laboratory proved for the first time that titanium dioxide's surface defects shelter chemicals from decays caused by ultra-violet light ("Inhibitive Influence of Oxygen Vacancies for Photoactivity on TiO2(110)"). The defects are tiny gaps created when oxygen atoms are missing from the surface of this popular catalyst. Conventional wisdom says the vacancies are more active than the surface. The team showed the opposite is true for photooxidation. The carbon-based carboxylic group, trimethyl acetate (TMA), remains intact if bound in the vacancies while it readily decomposes at regular, or non-vacancy, sites on the surface.
|
|
"What's recognized generally is that oxygen vacancy defects are often the most reactive sites in catalysis," said Dr. Igor Lyubinetsky, a surface physicist working on the study as part of PNNL's Chemical Imaging Initiative. "We found that in photochemistry, the vacancies play the opposite role. They block the photo-oxidative reaction."
|
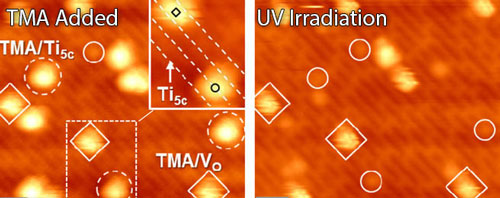 |
| For the first time, scientists were able to determine that oxygen vacancies on titanium dioxide locally inhibit the material's ability to do photochemistry. Here a scanning transmission microscopy image shows the surface after TMA was added (left) and after ultra-violet irradiation.
|
|
Why It Matters
|
|
Titanium dioxide is used in dye-sensitized solar cells, self-cleaning windows, and other devices. While it has been studied for about 40 years, titanium dioxide's behavior at an atomic level is not well defined. By understanding how the atoms interact, scientists could open the door to fine tuning the material. For example, they could make it absorb more light, creating more efficient solar cells. They could also take the self-cleaning window technology and apply it to create a highly selective material that decomposes only the targeted pollutants in a complex solution.
|
|
"If you look toward applied research, this finding could do some very interesting things regarding selectivity," said Dr. Michael A. Henderson, who has been leading photochemistry at PNNL for years.
|
|
Methods
|
|
When the right wavelength of light is shined on titanium dioxide, it absorbs a photon or packet of the light and creates electron and holes. A hole is a mathematical construct for the absence of an electron; that is, the lack of an electron where one could exist. The holes are vital to the decomposition of TMA, a stand-in for various pollutants and chemicals, absorbed onto the surface of titanium dioxide.
|
|
Starting with titanium dioxide, the team introduced oxygen vacancies, spots where oxygen atoms were removed from the surface. They obtained high-resolution images of the surface using the ultra-high vacuum scanning tunneling microscope at DOE's Environmental Molecular Sciences Laboratory. They found that when the TMA bonded to the surface was exposed to light, it oxidized or decomposed. The catalyst behaved as expected. However, when some species settled inside an oxygen vacancy, it remained intact. The catalyst was completely inhibited by the oxygen vacancy defects.
|
|
To eliminate the possibility that the reaction was suppressed because of structural changes in either the surface or the TMA, the team used density functional theory calculations on EMSL's supercomputer and electron energy loss spectroscopy experiments. They determined that the lack of reactivity was not because the TMA is deformed when it absorbed on the vacancies, and there was no change in the vacancy's atomic arrangement or electronic structure.
|
|
With further analysis, the team showed that the cause was two excess electrons that remain near the oxygen atom vacancy. The electrons jump nearby and are distributed around the vacancy. When the light strikes the crystal and starts to create electrons and holes, these extra electrons recombine with holes and just generate heat. In creating the heat, the photo-generated hole surrenders the opportunity to break apart the TMA, leaving it to rest unchanged in the vacancy.
|
|
"The extra electrons effectively screen vacancies from reaction," said Dr. Zhi-Tao Wang, first author on this study.
|
|
What's Next?
|
|
The role of oxygen vacancies on titanium dioxide is an area of ongoing research at PNNL. "This is just the first example," said Lyubinetsky. "We don't believe it will matter if it is TMA photodecomposition or other hole-induced reactions involving molecules in the vacancies. This study should have broad application in the scientific community."
|

