| Jul 30, 2013 |
First quantitative insights into electron transfer from minerals to microbes
|
|
(Nanowerk News) Scientists have gained the first quantitative insights into electron transfer from minerals to microbes by studying that transfer in a nature-inspired, protein and iron-based nanoparticle system. Iron plays a crucial role in environmental biogeochemistry. It readily exchanges electrons with microbes, transforming from more soluble Fe(II) to less soluble Fe(III).
|
|
By studying that exchange, researchers better understand iron cycling in the environment and how iron cycling, carbon cycling, and microbial activities are connected.
|
|
For their studies ("Fe3–xTixO4 Nanoparticles as Tunable Probes of Microbial Metal Oxidation"), the research team used ‘tunable’ Fe3–xTixO4 nanoparticles in which the Fe(II)/Fe(III) ratio is controlled by replacing Fe atoms with Ti atoms in the nanoparticle lattice—the more Ti, the more Fe(II).
|
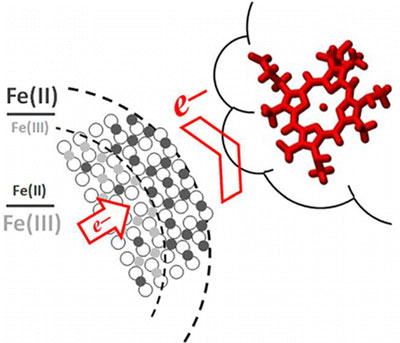 |
| The first quantitative insights into electron transfer from minerals to microbes show that the cytochrome, MtoA, extracts electrons from structural Fe(II) in nanoparticles from the outside in, leaving behind Fe(III) and not damaging the crystal structure. The higher the Fe(II)/Fe(III) ratio in the nanoparticles, the faster the electron transfer.
|
|
The team exposed nanoparticles with different Fe(II)/Fe(III) ratios in solution to purified MtoA, an iron-oxidizing cytochrome from the water-dwelling microbe, Sideroxydans lithotrophicus ES-1. They detailed the oxidation kinetics of the nanoparticles by the cytochrome in real time, in situ, and with Ångström-level resolution using a novel tool set. Stopped-flow spectrometry at EMSL was used to monitor protein absorbance changes, which were used to calculate electron transfer reaction kinetics. Micro-X-ray diffraction at Environmental Molecular Sciences Laboratory (EMSL) showed changes in the Fe(II)/Fe(III) ratio in the nanoparticle lattice. X-ray absorption and magnetic circular dichroism spectroscopies with synchrotron resources at the Advanced Light Source revealed changes in the Fe(II)/Fe(III) ratio as well as in magnetic properties at the nanoparticle-cytochrome interface.
|
|
The team found that MtoA extracted electrons from structural Fe(II) in the nanoparticles starting at the surface then continuing to the interior, leaving behind Fe(III) and not damaging the crystal structure. Also, the higher the Fe(II)/Fe(III) ratio in the nanoparticles, the faster the electron transfer. The team’s novel system can be adapted to study other key players in geochemistry, such as electron-transfer proteins in Geobacter and Shewanella as well as iron-containing minerals, such as hematite.
|
|
Fundamental studies such as these have broad implications—from improved biogeochemistry and earth science predictive models to understanding the impact of using nanoparticles for biotechnological applications, such as bioremediation and energy generation.
|

