| Aug 03, 2013 |
Shining laser light on protein stability measurements
|
|
(Nanowerk News) Researchers at the London Centre for Nanotechnology (LCN) have developed a method for measuring the stability of proteins using vastly reduced amounts of sample in a much shorter timeframe.
|
|
Proteins are the fundamental building blocks of all life on earth. These are the physical components that are decoded from genes into the myriad forms of life seen around us.
|
|
Understanding the stability of proteins is central to understanding their structure, function and role in diseases and in determining therapeutic agents. It also has large ramifications in both designing industrial processes where a protein is used to produce valuable products and in gauging how well a drug binds to its target (which will almost invariably itself be a protein).
|
|
The LCN study, published in Scientific Reports ("Optically Induced Thermal Gradients for Protein Characterization in Nanolitre-scale Samples in Microfluidic Devices"), shows that the stability of protein can be measured by slowly subjecting it to an increasing amount of heat, delivered by an Infrared laser beam in a ultra-small volume glass tube, replicating the conditions found within the bioreactors of industrial processes.
|
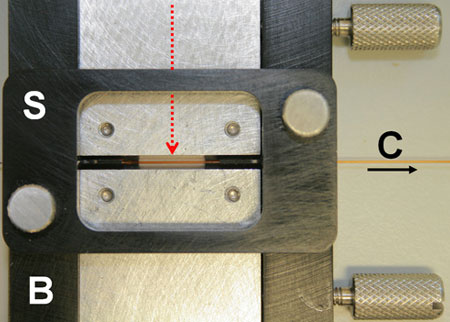 |
| Figure: A custom-made butterfly stage (B) aligns an IR laser via embedded optical fibre (red arrow) to a micro-capillary (C, arrow shows flow direction) clamped with a setting tool (S).
|
|
The laser heating directs heat at a point along the glass tube which creates a gradient of temperature along the sample. This allows the measurements of protein stability over a range of temperatures to be made concurrently. With traditional methods the temperature must be raised between measurements, therefore requiring more time and a larger sample size.
|
|
Numerical modelling gives a good account of how the all-optical device (shown in the Figure - note the complete absence of electrical connections) with its protein sample behaves, a validation which enables computer-aided design and engineering of new products based on the same underlying principles.
|
|
Measurements of protein stability are also necessary for the drug development process; the targets of drugs are almost always proteins. For a drug to be effective it is important that it binds strongly to its protein target. If a range of drug candidates are bound to a protein target, the heat required for the two to fall apart is a measure of how tightly they were originally bound together, which is something that can be established using the LCN technology.
|
|
The improved technique leads to much more rapid protein stability screening, with a hugely reduced amount of sample being required. This implies substantial cost savings when integrated into industrial and pharmaceutical processes.
|
|
M. Sagar Dodderi, one of the researchers in this study said, "This first quantitative study on optically induced protein denaturation paves the way towards miniaturized devices for a wide range of healthcare and biotechnology applications. These include personalised medicine and environmental biosensing for pathogens and in biomanufacturing"
|
|
The LCN method reported in the journal article is the subject of a patent application filed in conjunction with UCL Business, the technology transfer office of UCL.
|

