| Oct 07, 2013 |
Guinness World Record for the discovery of the thinnest glass
|
|
(Nanowerk News) It is only a couple of molecules thick, and could not be thinner: the sheet of glass that scientists at the University of Ulm and Cornell University have discovered by accident. This discovery has now been acknowledged as a world record with an entry in Guinness World Records 2014.
|
|
“Although glass is indeed transparent, the individual silicon and oxygen atoms can be made visible under the electron microscope”, explains Ute Kaiser. The Professor of Experimental Physics directs the electron microscopy group of materials science at the University of Ulm. She is still fascinated by this very special journey of discovery, which has taken more than one year: “Step by step we have, through our experiments and reflections, unravelled the secrets of the material, and this was unbelievably exciting. A real science thriller.”
|
|
Simon Kurasch, at that time still a graduate student studying for a doctorate under Ute Kaiser at the University of Ulm, was investigating the atomic structure of a graphene sample under an extremely high-resolution transmission electron microscope. This is a monolayer comprised only of carbon atoms whose hexagonal atomic structure is reminiscent of a honeycomb and for the discovery of which the Nobel Prize was awarded in 2010. In actual fact, this had been a routine investigation for the physicist.
|
 |
| The physicists from Ulm University, Prof. Dr. Ute Kaiser and Simon Kurasch work with the highest-resolution transmission electron microscopes, where the discovery was made.
|
|
But on closer examination the young researcher discovered a previously unseen and completely unexpected structure: “It is both wonderfully ordered and at the same time completely chaotic” is how Kurasch describes this chance finding. An extremely thin layer of an unknown substance had formed on the graphene. Inquiries made to the Max-Planck-Institute for Solid State Research in Stuttgart, which had produced the graphene on copper film in a quartz glass-lined furnace according to a standard method, prompted incredulity. The team of researchers there, organised around the solid state nanophysicist Dr Jurgen Smet, was initially unable to make any sense of this finding.
|
|
The Ulm physicist turned to Physics Professor David Muller, her colleague in science over many years and the Director of the Kavli Institute for Nanoscale at Cornell University (NY). Perhaps his team in New York State could contribute very high-resolution imaging and spectroscopic data on the chemical nature of the material. Muller agreed.
|
|
A four-strong German-American team of researchers was set up, consisting of the two Ulm scientists together with Muller and Pinshane Huang, his graduate student studying for a doctorate; the team had been jointly conducting research at Cornell University for some time. Muller soon pointed the result to a silicon-oxygen compound. Further clues were urgently sought to explain the precise chemical composition of the mysterious material. It turned out that the ultrathin layer consisted of silicon dioxide, i.e. glass. With its special atomic structure, this amorphic material is still baffling the scientific community. As a result, it was still a question for the international group of physicists of clarifying the molecular configuration of the sheet of glass.
|
|
Ute Kaiser therefore sought the advice of her Finnish colleagues. Dr Arkady Krasheninnikov of the Aalto University of Helsinki, a proven expert in calculating the stability of atomic bonds, was ultimately able to show with his colleagues that silicon dioxide takes on the most stable possible configuration in two layers, i.e. a ‘double layer’. “So it turned out by all analytical and theoretical results together that we had found the thinnest conceivable sheet of glass, which was thus actually two-dimensional”, according to the team. Scientists were thus for the first time able to gain a clear insight into the atomic structure of this special material.
|
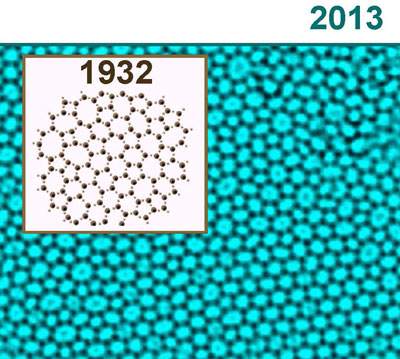 |
| The image shows a model of the atomic structure of SiO2 suggested by W.H. Zachariasen back in 1932. In the back we see the experimental TEM image of the year 2013 taken by Simon Kurasch. The similarities are very obvious. The dark contrast corresponds to the Si atoms.
|
|
Glass is an “amorphous” material, which, though having the physical properties of a solid, exhibits in its atomic structure properties of both liquids and solids. “If electron microscope images are examined, one can see a layer of irregular and disparate polygons. It looks like a rag rug comprised mostly of pentagons, hexagons, heptagons and octagons”, explains the Ulm electron microscopy expert Ute Kaiser. “Using our results, we were surprisingly able to confirm a therapy formulated by W.H. Zachariasen back in 1932”.
|
|
The network hypothesis formulated by the Norwegian-American physicist concerning the atomic structure of glass posited, in broad terms, that glass is in its basic atomic structure – consisting of SiO4 tetrahedrons – similar to crystal, with the only difference being that these tetrahedrons are connected to one another much more randomly than in very regularly organised crystal, so that the arrangement appears much more irregular.
|
|
This scientific “thriller”, the results of which were published in Nano Letters back in 2012 ("Direct Imaging of a Two-Dimensional Silica Glass on Graphene"), also had a double happy ending for the international team of researchers. Not only did the team manage to identify the thinnest conceivable glass but it also solved a previously unsolved materials science puzzle. Lastly, the issue of the atomic structure of glass is not only one of the great questions of inorganic science but also one of the greatest analytical problems of physics. The entry in the Guinness book is thus well deserved.
|


