| Feb 19, 2015 |
Classical nova explosions are major lithium factories in the Universe
|
|
(Nanowerk News) A team of astronomers from National Astronomical Observatory of Japan (NAOJ), Osaka Kyoiku University, Nagoya University, and Kyoto Sangyo University observed Nova Delphini 2013 which occurred on August 14, 2013. Using the 8.2-meter Subaru Telescope High Dispersion Spectrograph (HDS) to observe this object, they discovered that the outburst is producing a large amount of lithium ("Explosive lithium production in the classical nova V339 Del (Nova Delphini 2013)").
|
|
Lithium (Li) is a key element in the strudy of the chemical evolution of the universe because it likely was and is produced in several ways: through Big Bang nucleosynthesis, in collisions between energetic cosmic rays and the interstellar medium, inside stellar interiors, and as a result of novae and supernova explosions. This new observation provides the first direct evidence for the supply of Li from stellar objects to the galactic medium. The team hopes to deepen the understanding of galactic chemival evolution, given that nova explosions must be important suppliers of Li in the current universe.
|
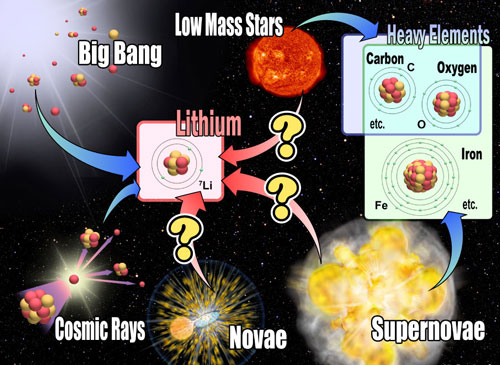 |
| Nucleosynthesis in the universe. Heavy elements such as C, O, and Fe are mainly produced in stellar interior and/or supernova. On the other hand, Li might be produced in many other ways: in the Big Bang, galactic cosmic ray collisions. Li production in stellar originating objects has not been confirmed yet by observations, as designated by "?" marks. (Credit: NAOJ)
|
|
Lithium: the Key to Understanding the Nucleosynthesis in the Universe
|
|
The universe consisted primarily of hydrogen (H) and helium (He) immediately after the Big Bang except for very small amounts of Li. Since there are other elements heavier than H and He in the universe now, astronomers want to understand how the heavy elements -- such as carbon (C), oxygen (O), and iron (Fe) (which are present in our bodies) -- are produced. Such heavy elements are mainly produced in stellar interiors or supernovae. Then, they are supplied to the interstellar medium as seed materials for next generation of stars.
|
|
Li is the third lightest element following H and He, and is familiar to us as the base materials for the Li-ion batteries used in PCs, smart phones, eco-cars, etc. Big Bang nucleosynthesis produced a very small amount of Li. Collisions between atomic nuclei in the interstellar medium are also assumed to produce Li by breaking heavy elements' nuclei (e.g., C, O). Low-mass stars like the Sun, and events such as supernova explosions are also considered as condidates of Li production sites. Furthermore, scientists have been assuming that novae should also produce this element.
|
|
Because many sites and events can produce Li as described above, Li is the best indicator to probe the complete chemical evolution of the universe. Many scientists have studied this element by measuring the amount of Li found in various stars in our galaxy. This allowed them to estimate the amount produced through each process. Today, as a result of these indirect approaches, low-mass stars or nova explosions are thought to be the most important candidates for Li production in the current galaxy epoch. However, there have been no direct observations of the processes.
|
|
Nova Delphini 2013
|
|
On August 14th, 2013, the well-known Japanese amateur astronomer Koichi Itagaki found a bright new star in the constellation Delphinus. This star, which was named Nova Delphini 2013 (=V339 Del), was at magnitude 6.8 at discovery and peaked at 4.3 mag within two days. It was the first naked-eye nova since 2007, when V1280 Sco was found. About 40 days later, in September 2013, a team of astronomers observed the nova to investigate the materials expelled by the explosion. That is when they found that the nova produced a large amount of Li.
|
|
Nova Delphini 2013 is considered one of the "classical novae". These brighten when explosive nuclear reactions occur in materials accumulated on the surface of a white dwarf star in a close binary system. The nuclear reactions are thought to produce a different series of elements (compared to those produced in stellar interiors or supernova explosions). Li is assumed to be an element typically produced in such outbursts. Historically, no one has been able to get good observational evidence for its production in nova explosions.
|
|
Discovery of a Beryllium Isotope (7Be) to Form Lithium in Nova Spectra
|
|
When the research group observed Nova Delphini 2013 using the Subaru Telescope, they used the High Dispersion Spectrograph to discern the constituents of the expelled materials from the nova explosion at four epochs.
|
|
Absorption lines originating from many elements such as H, He, and Fe are identified in the observed spectra. Among them, there are sets of strong absorption lines in the ultraviolet (UV) range (wavelength ~313 nanometers) of the spectrum. Comparing these lines with other lines originating from H, calcium (Ca), and other elements, it turns out that they are originating from an isotope of beryllium (Be), 7Be, which is the fourth-lightest element in the universe.
|
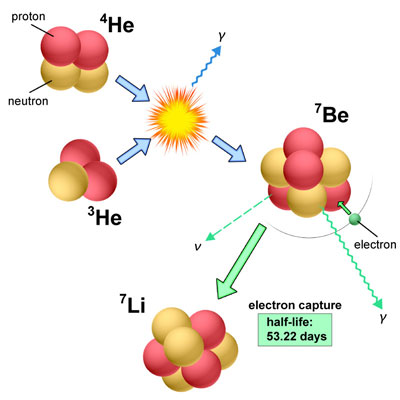 |
| Nuclear reactions to form 7Be, and then 7Li in classical nova explosions. At the time of an explosion, 3He and 4He are fused to form 7Be (blue arrows). Then, 7Be gradually decays into 7Li (via electron capture) in gas blobs blown away by explosive winds (green arrows). (Credit: NAOJ)
|
|
In a classical nova, the isotopes of He (3He) and plentiful 4He transferring from the companion are fused together to form radioactive 7Be in a very high-temperature environment on the surface of a white dwarf. This radioactive isotope decays to form an isotope of lithium (7Li) within a short time (half-life of 53.22 days). Because 7Li is very fragile in a high-temeperature environment, it is necessary to transport 7Be to a cooler region in order to enrich Li in the inteerstellar medium. Novae completely fill this requirment. Therefore, they are assumed to be strong condidates as suppliers of Li in the universe.
|
|
This discovery of 7Be within 50days after the nova explosion means that this explosion is actually producing a large amount of 7Li formed from 7Be. Because 7Be is found in the gas blobs blown aways from the central region of the nova at high velocities (~1000 km/s), 7Li formed from this 7Be should not be destroyed in a high-temperature environment. This 7Li spreads into interstellar space, and will be included in the next generation of stars. It is found that the 7Be abundance in the gas blobs estimated from the strengths of their absorption lines is comparable to that of Ca. This amount of 7Be (= 7Li) should be quite large, given that Li is known as a very rare element in the universe.
|
|
Impact of this Research
|
|
The amount of Li rapidly increases in the galaxy in the current epoch, where the amounts of heavy elements have increased. Therefore, it has long been speculated that low-mass stars with longer lifetimes should be among the major suppliers of Li in the universe. Because nova explosions occur in binary systems evolved from such low-mass stars (especially 3He-rich companion, which is necessary to produce 7Be), they are strong candidates as Li suppliers. The observations made using the Suberu HDS provide the first strong evidence to prove that novae produce significant amounts of Li in the universe. This discovery confirms the chemical evolution model from the Big Bang to the present universe, as predicted by scientists.
|
|
Furthermore, the observed amount of Li produced in this nova explosion is proven to be highter than predicted by theoretical estimates. Nova Delphini 2013 shows rather typical characteristics of classical novae. If other novae also produce a large amount of Li as Nova Delphini 2013 did, nova explosions must be recognized as very major Li factories in the universe. In near future, more observations of other nova explosions will provide much clearer model of Li evolution.
|


