| Posted: Nov 07, 2006 | |
Molecular imaging as a step towards personalized medicine |
|
| (Nanowerk Spotlight) Conventional diagnostic imaging is mainly based on morphological contrast that is a result of different general tissue characteristics. Molecular imaging is a new approach for detecting diseases much earlier, visualizing biological processes at the cellular and molecular level in living organisms, and detecting changes in biochemistry. Corresponding molecular markers appear in quite low concentrations. Hence, the imaging technique must be very sensitive. Magnetic resonance imaging (MRI) has some significant advantages in terms of using non-ionizing radiation (in contrast to x-rays) and giving high resolution tomographies for any arbitrary position and orientation. However, conventional MRI suffers from inherent low sensitivity. | |
| A new method, using xenon as the signal source, was developed by researchers in California and will make MRI an important technique in molecular imaging, offering a huge potential for specific detection of disease markers. The new technique allows detection of signals from molecules present at 10,000 times lower concentrations than conventional MRI techniques. Called HYPER-CEST, for hyperpolarized xenon chemical exchange saturation transfer, this new technique could become a valuable tool for medical diagnosis, including the early detection of cancer. | |
| In vivo molecular imaging requires specific sensor molecules that exclusively target a biomolecule of interest. Such sensors are difficult to develop for MRI with sufficient sensitivity because the technique detects tiny magnetizations of molecules that are exposed to a magnetic field. There are many molecular imaging agents – MRI contrast agents, PET and SPECT probes, fluorescence probes like quantum dots – and there is already a huge variety of targeting groups used for these probes. | |
| "The motivation for our work was to combine two clever detection methods to boost the MRI sensitivity" Leif Schröder explains to Nanowerk: "First, our xenon biosensor concept makes use of transferring the molecular information onto nuclei that are encapsulated in molecular cages and that can be detected at very low concentrations because they are hyperpolarized. This means that they have a much larger detectable magnetization. Second, we used an indirect detection assay that makes optimized use of all the xenon atoms. One biosensor molecule can be used to label thousands of xenon nuclei and to detect a significantly increased signal." | |
| Schröder, a member of the Pines Lab at UC Berkeley, is first author of a recent paper, titled "Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor", that was published in the October 20, 2006 edition of Science. | |
| The xenon biosensor project is a cooperation between the Pines Lab and the Wemmer Group at Lawrence Berkeley National Laboratory that started several years ago. The first paper on this biosensor concept was published in PNAS in 2001. Subsequently, several important steps towards the imaging application were presented, including work on optimized xenon delivery and the first one-dimensional imaging profiles. | |
| Medical imaging technologies are advanced in providing high-resolution images but they often lack the image contrast needed to, for example, distinguish healthy from diseased tissue. Christian Hilty, one of Schröders co-authors and now at Texas A&M University, expects the development of the xenon biosensor to allow highly sensitive magnetic resonance imaging that is specifically targeted to disease indicators. "The detection of specific diseases, such as cancer, at an early stage and with high fidelity, becomes possible" he says. | |
| Xenon offers the resolution of conventional MRI agents but much higher sensitivity, even approaching SPECT and PET. A huge advantage for hyperpolarized xenon is its near zero-background. | |
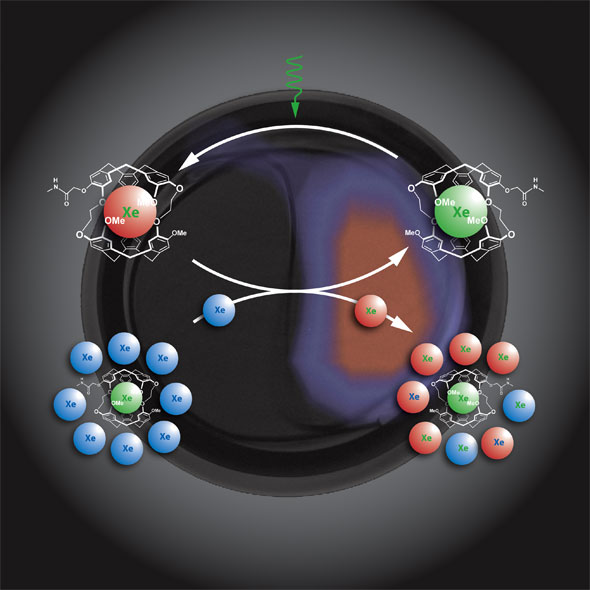 |
|
| Amplifying the cage-related magnetization using HYPER-CEST. Selective saturation of biosensor-encapsulated Xe (green) and subsequent chemical exchange with the free Xe (blue) allows accumulation of depolarized nuclei (red). This procedure corresponds to continuous depolarization of cage-related magnetization that can be measured indirectly after several cycles by the difference between initial and final bulk magnetization. (Image: Pines Lab) | |
| "There is no xenon naturally present in the body, so we don't have to fish out a small change in signal with a high background like other MRI agents" says Tom Lowery, one of the co-authors. "Its more like PET where you have a small signal with no background. Unlike PET and MRI agents, multiple xenon biosensors can be used to detect different targets at the same time, much like different colors of quantum dots can simultaneously report different analytes but that xenon agents can be detected deep within the body." | |
| Schröder describes the ideal 'smart' molecular imaging contrast agent: It should have a high, exclusive affinity to one specific target molecule and should be 'activated' somehow when the binding event takes place. The readout technique should have no background signal and should allow for signal amplification to decrease the detection limit. | |
| "The xenon biosensor HYPER-CEST approach fulfills all these requirements" he says: "The targeting unit of the biosensor reports a binding event with biochemical specificity. Hence, each target for which an affinity agent is known (e.g. a ligand or antibody) could be revealed by a 'tailored' biosensor. The xenon nuclear magnetic resonance signal from nuclei inside the biosensor cage differs significantly from the signal of free dissolved xenon. This principle 'activates' the to-be-detected nuclei in pathological regions where the biosensor accumulates. Finally, HYPER-CEST detects amplified signal changes that originate only from a few active centers but that can be transferred to many more probe nuclei. Since xenon is no endogenous element, the obtained images have no background signal – an ideal condition like in the case of PET, but with the MRI advantage of not depending on high-energy ionizing radiation." | |
| Thanks to HYPER-CEST, molecular imaging could now benefit from all the other MRI advantages like high spatial and temporal resolution and unlimited penetration depth to detect cancer or cardiovascular diseases at a very early stage and to develop a personalized treatment in direct response to changes on the molecular level. This would also allow for much easier follow-up studies of novel therapeutic approaches. The combination with conventional MRI techniques would give a comprehensive imaging tool that includes information on functional parameters like perfusion, diffusion etc. | |
| "Since this technique will be quite valuable for cell tracking in vitro, our next crucial step will be the biosensor-based detection of cells carrying a specific molecular target" says Schröder. "The technique has some potential for further optimization that we want to use to push the detection limit into the nano- or picomolar range for those in vitro applications." | |
| Future directions will focus on the in vivo implementation of the technique, i.e. demonstration of molecular imaging with the xenon biosensor in living animals and, ultimately, in humans. | |
| Although molecular imaging can be used for diagnosing cancer, ultimately it is a very important tool to realize the dream of personalized medicine. That is, medical treatment tailored not just to symptoms, but to the biochemical profile of an individual's disease state (gene expression, proteome, dominant metabolic pathways, etc). | |
| Lowery explains: "There are many diseases, certain types of cancer being some of them, that have sub-types whose proper medical treatments are drastically different. Doses that may cure one sub-type can be lethal to others. Distinguishing these in an efficient way for diagnosis and drug development is a very important challenge. There are many new detection tools for ex-vivo, fast, real-time medical profiling (capillary electrophoresis, proteomics with mass spec, etc) but molecular imaging offers what those techniques cannot: spatial and biochemical information. That is where hyperpolarized xenon agents can really contribute." | |
| The researchers are now working on antibody-targeted probes for a cell-based version of their experiments. The next major step is to do xenon biosensor molecular imaging in a small animal model. After the first in vivo xenon biosensor images have been demonstrated, development can become serious for diagnostic applications for humans; a prospect that probably is some 5 - 10 years down the road. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
