| Posted: Apr 15, 2009 | |
Nanomedicine's use of targeted delivery vehicles will revolutionize cancer diagnosis and therapy |
|
| (Nanowerk Spotlight) Applications with targeted nanoparticles are expected to revolutionize molecular imaging and cancer therapy. Cancer researchers are looking to nanoparticles as agents in various nanomedicine applications – as a drug carrier capable of localizing, attaching to, and directly releasing drugs into the cell nucleus; as a cellular biomarker; and as imaging and therapy agent in cancer medicine. | |
| In today's chemotherapy, together with radiation and surgery the main tools against cancer, doctors are pumping the patient full of cytotoxic drugs, that go everywhere in the body, with the hope that enough of the drugs reach the cancer cells and target their nuclear DNA to damage it or destroy the cell. Not only do chemotherapeutic techniques have a range of often serious side effects, mainly affecting all the fast-dividing cells of the body, it also has been shown that often less than 1% of the administered drug molecules enter tumor cells and bind to the nuclear DNA. | |
| Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. Dividing cancer cells acquire genetic changes at a high rate, which means that the cells in a tumor that are resistant to a particular drug will survive and multiply. The result is the re-growth of a tumor that is not sensitive to the original drug. | |
| Cancer researchers are therefore experimenting with nanoparticles as both contrast agent and drug carrier capable of pinpointing and destroying individual cancer cells. | |
| Targeted nanoparticles consist of a metallic or organic core conjugated with a biomolecule of interest. To be able to navigate nanoparticles to a desired target (i.e. a specific cancer cell), they need the property of specific target recognition. Depending on the type of cancer that is to be targeted, researchers choose biomolecules that show high affinity toward these specific tumor cells. Think of these biomolecules as a navigation aid to transport nanoparticles to the cancerous site or organ of interest. | |
| Antibodies and peptides are the most commonly used target vectors for this purpose. However, antibodies are highly immunogenic – i.e. they trigger the body's defense mechanism, which can lead to harmful side effects – and they have poor in vivo diffusion characteristics. Peptides on the other hand show beneficial attributes such as rapid blood clearance, increased diffusion rates in tissue, and low immunogenicity. | |
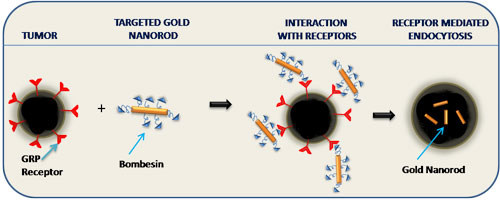
| As part of their overall goal of developing target-specific gold nanoparticles for treatment of cancers, Raghuraman Kannan, Kattesh Katti, and colleagues at the University of Missouri have carried out a systematic investigation on the design and development of targeted gold nanorods. A recent result of this work has been the design of a novel peptide-based nanovector for carrying drug payloads to cancer sites. This vector design exploits the high affinity that bombesin peptides show towards gastrin-releasing peptides (GRP). Since GRP receptors are overexpressed in many cancers, the researchers hypothesized that bombesin peptide can act as a vehicle to deliver gold nanorods specifically to certain tumor cells. | |
| "In our recent work we have conjugated gold nanorods to bombesin peptide" Raghuraman Kannan tells Nanowerk. "We have demonstrated that our gold nanorod-bombesin conjugate can internalize within GRP receptor expressing tumor cells. We believe that these nanovectors possess realistic clinical applications." | |
| Kannan is an assistant professor in the Department of Radiology, and director of the Nanoparticle Production Core Facility at the University of Missouri-Columbia. Together with his collaborators Nripen Chanda, Ravi Shukla, and Kattesh V. Katti, he believes that the selective cancer-targeting capabilities of gold nanorod-bombesin conjugates and the associated in vitro stabilities provides unprecedented opportunities for the utilization of these vectors in molecular imaging and therapy of cancer. | |
 |
|
| Schematic of how the gastrin releasing protein receptor specific gold nanorods work. (Image: Dr. Kannan, University of Missouri) | |
| Since these nanovectors show highly selective targeting to cancer cells without harming healthy cells (they don't show any affinity towards them), their potential in real-world nanomedicine applications is considerable. Future applications could include either imaging techniques where the gold nanorods can be used as contrast agents for computer tomography and optical coherence tomography imaging, or in theranostics, where again the gold nanorods are used as contrast agent for imaging and subsequently as tumor cell killers using a photo-thermal modality. | |
| The team has reported their findings in the April 7, 2009 edition of Nano Letters ("Gastrin Releasing Protein Receptor Specific Gold Nanorods: Breast and Prostate Tumor Avid Nanovectors for Molecular Imaging"). | |
| Stability and affinity of the nanoparticle towards tumor cells are two important parameters in evaluating the in vivo applicability of an agent. The degree of stability is key in determining whether an agent can be used for in vivo purposes. The degree of affinity of conjugates to tumor cells determines whether it is truly going to attach to the targeted tumor cells. | |
| "We investigated the two parameters for our gold nanoconjugates" says Kannan. "With regard to stability, we established that using thioctic acid we can add extraordinary strength to the conjugate, making it stable enough for in vivo purposes. With regard to affinity, our studies provide quantitative data precisely estimating the affinity of conjugates toward receptors on cells, which in the case of our conjugates is very high." | |
| Kannan explains that one of the most important factors in the development of a nanoparticle as a drug candidate is the so-called IC50 value – the half maximal inhibitory concentration. This value provides crucial information on the effectiveness of a compound in inhibiting a biological or biochemical process. | |
| "It is surprising that there are very few well defined methods available for the estimation of IC50 values for targeted nanoparticles since estimation of the IC50 value is considered step 1 for developing targeted drugs," says Kannan. "In our recent work, we have developed a novel method for the estimation of IC50 value for gold nanoparticles. This quantitative estimation in micrograms show effective the nanoparticle towards particular receptors. Using this number or value we can compare the efficacy of different nanoconjugates." | |
| Overall, there are several interesting findings coming out of the University of Missouri team's work: 1) From a chemistry point of view, it is the use of thioctic acid skeleton as a linker to conjugate gold nanorods with peptide; 2) from a stability point of view it is that, again due to thioctic acid, the conjugate inferred a high level of stability to the tagged molecule with gold nanorods, and 3) from a drug efficacy point of view, it is the development of a novel method for the estimation of IC50 value for gold nanoparticles. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
