| Posted: Dec 05, 2006 | |
Nanotechnology advances the efforts to achieve artificial photosynthesis |
|
| (Nanowerk Spotlight) Artificial photosynthesis, using solar energy to split water generating hydrogen and oxygen, is often considered a 'Holy Grail' of chemistry which can offer a clean and portable source of energy supply as durable as the sunlight. It takes about 2.5 volts to break a single water molecule down into oxygen along with negatively charged electrons and positively charged protons. It is the extraction and separation of these oppositely charged electrons and protons from water molecules that provides the electric power. The photocatalytic splitting of water into hydrogen and oxygen using solar light is a potentially clean and renewable source for hydrogen fuel. Although massive efforts have been made in this area, many researchers have faced different types of challenges reaching from fundamental sciences to engineering. Titanium dioxide has been considered one of the most promising photocatalytic materials due to its relatively low cost, chemical stability, and photostability. However, the catalytic property of titanium dioxide is limited with ultraviolet (UV) regions which accounts for only ∼4% of the incoming solar energy and thus renders the overall process impractical. Tungsten trioxide has been recently focused on as a new photoanode material, or as a mixture material with titanium dioxide, for water splitting because the tungsten trioxide can offer relatively small band gap (∼2.5 eV) and corrosion stability in aqueous solution. Although tungsten trioxide has shown great potential such as photooxidation of water with visible light and high photocurrent with nanocrystals, the quantum yield is still low. In new research, titanium oxide nanotubes coated with tungsten oxide were prepared to harvest more solar light for the first time. The tungsten trioxide coatings significantly enhanced the visible spectrum absorption of the titanium dioxide nanotube array, as well as their solar-spectrum induced photocurrents. | |
| Several efforts have been made to employ mixed tungsten trioxide (WO3) /titanium dioxide (TiO2) systems for enhancing the efficiency of electrochromic effects and the photocurrent in aqueous solution. Researchers at the Department of Chemistry and Biochemistry at the University of Texas at Austin had already demonstrated previously that carbon doped TiO2 nanotube arrays with high aspect ratio improve the photocurrent densities ("Novel Carbon-Doped TiO2 Nanotube Arrays with High Aspect Ratios for Efficient Solar Water Splitting"). | |
| In a recent study, the researchers employ TiO2 nanotubes coated with WO3 as a photoanode for the first time with the photoelectrochemical data obtained showing an increase in solar harvesting efficiency. This nanocomposite material can be used as efficient and stable solar-driven photoanode for oxidation of water. | |
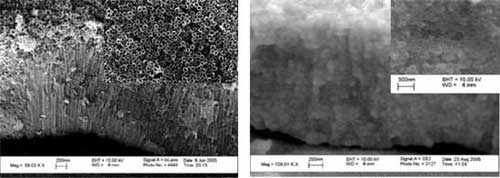 |
(left) SEM images lateral view and top view of TiO2 nanotubes. b SEM images lateral view and top view of WO3/TiO2 nanotube nanocomposite electrodeposited for 5 min.(Reprinted with permission from the American Institute of Physics) |
| This new research was reported in a recent paper in Applied Physics Letters ("Photoelectrochemical water splitting at titanium dioxide nanotubes coated with tungsten trioxide"). | |
| For their experiments, the researchers coated a TiO2 nanoarray of approx 2µm length with WO3 through electrochemical deposition. The resulting nanocomposite still has a nanotubular structure, however, the nanocomposite materials are fully filled with WO3 when the deposition time is longer than 20 min. They found that the maximum photocurrent of the WO3/TiO2 nanotube nanocomposite was increased compared with that of the TiO2 nanotubes due to additional light absorption. The scientists point out that for the applications of TiO2 and WO3 as a photoanode in a photoelectrolysis cell under solar light illumination, it is particularly important to introduce a large interface area where photoinduced charge transfer by excitons into separated electrons and holes can efficiently occur. | |
| The future work in this field will be focused on searching for new methods to grow more regular shaped nano-structures and different coating materials with lower band gap and stabilities in aqueous solution. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
