| Posted: Jan 12, 2007 | |
Medical nanotechnology: Killing cancer with gold nanobullets and nanobombs |
|
| (Nanowerk Spotlight) Thermolysis (from thermo- meaning heat and -lysis meaning break down) is a chemical process by which a substance is decomposed into other substances by use of heat. | |
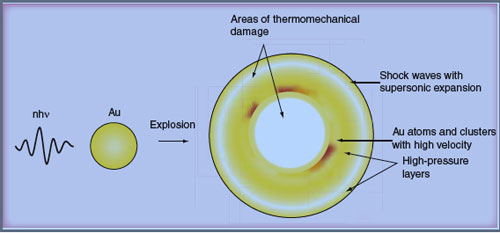
| In photothermolysis the transfer of laser energy is used to generate the required heat. And finally, nanophotothermolysis is the process where nanoparticles, when irradiated by short laser pulses, get hot so quickly that they explode. This thermal explosion of nanoparticles (nanobombs) may be accompanied by optical plasma, generation of shock waves with supersonic expansion and particle fragmentation with fragments of high kinetic energy, all of which can contribute to the killing of cancer cells they are attached to. | |
| By engineering the laser wavelength, pulse duration and particle size and shape, this technology can provide highly localized damage in a controlled manner, potentially varying from a few nanometers (for DNA) to tens of microns (the size of a single cancer cell) without damaging the surrounding tissue. | |
| Researchers have already shown how gold nanoparticles could be used in nanotechnology cancer diagnostics by binding them to malignant cells (see for instance Surface Plasmon Resonance Scattering and Absorption of anti-EGFR Antibody Conjugated Gold Nanoparticles in Cancer Diagnostics: Applications in Oral Cancer). | |
| Many cancer cells have a protein, known as epidermal growth factor receptor (EGFR), distributed widely on the outside of their cell membranes. In contrast, healthy cells typically do not produce much of this protein. By attaching gold nanoparticles to an antibody for EGFR (anti-EGFR), researchers were able to get the nanoparticles to specifically attach themselves to the cancer cells. | |
 |
|
| Gold nanoparticles stick to cancer cells and make them shine. (Source: Dr. Ivan El-Sayed, Georgia Tech) | |
| Gold nanoparticles are the most promising candidates for photothermolysis since they are strong absorbers, photostable, nontoxic, easily conjugated to antibodies or proteins and have adjustable optical properties. | |
| A recent review in Future Medicine (Laser-induced explosion of gold nanoparticles: potential role for nanophotothermolysis of cancer) gives a good overview of the current state of nanophotothermolysis. | |
| One of the pioneering works to develop methods for selective cell targeting based on the use of lasers and light-absorbing nanoparticles was done in 2003 ("Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles"). | |
| A method for selective laser killing of bacteria targeted with light-absorbing gold nanoparticles conjugated with specific antibodies was described in 2005 (Photothermal Nanotherapeutics and Nanodiagnostics for Selective Killing of Bacteria Targeted with Gold Nanoparticles). | |
Nanotechnology to kill cancer cells |
|
| Various killing mechanisms for cancer cells have been proposed. The one most commonly discussed, that has become an extensive area of research, is based on the formation of a bubble around the overheated gold nanoparticle in a liquid environment (see for instance Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles); generation of acoustic and shock waves; or protein inactivation. In particular, gold nanoclusters attached to a cell membrane can lead to dramatic increases in bubble formation efficiency, resulting in more severe cancer cell damage at a laser strength that is safe for normal tissue. | |
 |
|
| Laser-induced thermal explosion of a gold nanoparticle. (Source: Future Medicine) | |
| For environments with a lack of sufficient amount of liquid for efficient bubble generation, such as bones or dense solid tumors, other killing mechanisms are being sought. For instance, one is a 'gold atom bullet' that moves from the explosion zone with kinetic energy sufficiently large to mechanically damage the surrounding cellular structure. Another is the melting of cell walls by hot gold nanoparticles and subsequent destruction of the cell by gold nanoclusters. | |
| Detailed theoretical modelling needs to go into estimating the threshold laser energy intensity and pulse duration required for the thermal explosion mode of gold nanoparticles. This has to take into account the size and shape of the gold nanoparticle, the size of the particle cluster, the location of the particles with regard to the cell wall, as well as the surrounding environment and other possible parameters such as the desired bubble size or strength of shock wave. Theoretical simulations aimed at finding the optimal single-particle and cluster structures to achieve its maximal absorption, which is crucial for PT therapeutic effects (Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters). | |
| Several research groups are leading the pioneering work on developing the great potential of photothermal therapy for the selective treatment of cancer cells, bacteria, viruses, and DNA targeted with gold nanospheres, nanoshells, nanorods, and nanosphere clusters; among them: Halas Nanophotonics Group at Rice University, Vladimir Zharov at the Philips Classic Laser Biomedical Labs at the University of Arkansas, the El-Sayed research group at Georgia Tech. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
