| Posted: Nov 09, 2009 | |
Multifunctional nanotechnology device for integrated, cell-based nanotherapy |
|
| (Nanowerk Spotlight) One of the promises of medical nanotechnology is a drug-delivery nanodevice that can image, target, and deliver drugs to a specific cancer location inside the body and monitor and if necessary adjust the drug release at the target location. Researchers in Taiwan have now designed a nanodevice that comes pretty close to this vision. | |
| "We have successfully demonstrated that our multifunctional drug-delivery nanodevice – a polymer core/single-crystal iron oxide shell nanostructure bonded to a quantum dot – can image, target, and deliver drugs via remote control," Dean-Mo Liu, a professor in the Nano-Bioengineering Lab at National Chiao Tung University, tells Nanowerk. "The device shows outstanding release and retention characteristics via an external on/off manipulation of a high-frequency magnetic field. Furthermore, the quantum dot bonded to the nanodevice provides optical information for in situ monitoring of the drug release." | |
| In previous work reported last year, Liu and his colleagues have developed a new type of magnetic core/shell nanocapsule that allows active molecules to be expelled in the presence of a remotely controlled magnetic field ("Core/Single-Crystal-Shell Nanospheres for Controlled Drug Release via a Magnetically Triggered Rupturing Mechanism"). There, the team has shown that the release of the active molecules from the nanocapsules can be fine-tuned according to exposure time in the magnetic field. | |
| "However, we thought it would be desirable to further engineer the nanocapsules to monitor the release of active molecules at nanometric or cellular resolution," says Liu. "We also believed that a nanocapsule should be able to provide easily monitored, high-resolution optical information, viewable via simple spectroscopic methods, rather than conventional MRI imaging." | |
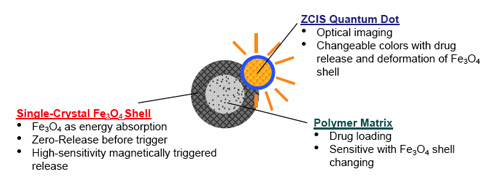
| On the basis of their previously developed core/shell drug-delivery nanocapsule – a polymer core covered with a thin layer of single-crystal iron oxide shell – the team designed a new drug-delivery vehicle by depositing zinc-copper-indium-sulphur (ZCIS) nanocrystals onto the nanocapsules' surface to form a nanoscale multifunctional platform. While the core/shell structure offers great therapeutic potential for controllable drug release, with dosage controlled via an external magnetic field, the quantum dots deposited on the surface of the magnetic nanoparticles are used for directly monitoring the release of the drugs. This is achieved by observing variations in the fluorescence intensity of the quantum dots. | |
 |
|
| Schematic drawing showing multi-functionalities of each compartment from as-designed nanodevices for nanoimaging, controlled drug release, and in situ motoring of drug release. (Reprinted with permission from Wiley-VCH Verlag) | |
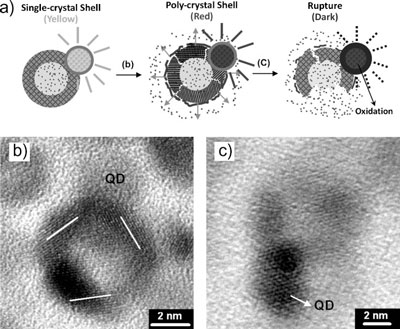
| After studying their nanocapsules in operation, Liu and his team propose the following operation mechanism for their nanodevice: After 60 seconds of exposure to an external magnetic field, the single-crystal nanoshell structure was subjected to lattice deformation which provided numerous nanoconduits that allow leaking of the encapsulated molecules to the environment. | |
| "In other words" says Liu, "for short-term induction, extremely small cracks or crevices were formed along the boundary regions of the thin iron oxide shell and are kept open by the presence of the magnetic field. Extended magnetic stimulus enlarged the nanocrevices until permanent rupturing of the shell occurred. At this time, the encapsulated molecules were released from the nanodevice easily and completely." | |
| Meanwhile, the energy structure of the ZCIS quantum dots altered considerably as a result of thermally induced photobleaching along with surface oxidation. This resulted in degeneration of the fluorescence intensity. | |
 |
|
| Schematic illustration of the nanodevice with a proposed mechanism for controlled release of the dye molecules, as well as the degeneration of fluorescence intensity of the ZCIS quantum dots. b) Shell vibration causing enlargement of the dimensions of nanocrevices along the deformed single-crystal shell, rendering dye release upon short-term magnetic stimulation. c) After long-term exposure, the deformed shell has received a sufficient amount of the energy to cause a final, permanent, mechanical rupture. Meanwhile, rapid surface oxidation altered the surface structure of the quantum dots, leading to substantial degeneration of the fluorescence intensity. (Reprinted with permission from Wiley-VCH Verlag) | |
| Liu says that one important concern for practical applications of this delivery device is whether the emission degeneration of the attached quantum dots occurs spontaneously without the presence of the magnetic field over a reasonable time period in a physiological environment. To examine this possibility, a simple test was performed by incubating the nanodevices in phosphate buffered saline solution for a time period of six hours, followed by measuring the photoluminescence emission spectrum at various time intervals. The team found that the resulting photoluminescence spectra of the quantum dots remained identical over the evaluation time span, i.e., the attached ZCIS quantum dot can act as a nanometric sensor to monitor the quantity of drug released from the drug-carrying nanocapsule. | |
| According to Liu, these nanodevices have great potential advantages as a cell-specific drug-delivery system for therapeutic applications. "With the in situ monitoring capability of the nanodevice, we believe that both target-oriented therapy and diagnosis can be integrated and managed within a single cell. We expect multifunctional nanometer-scaled platforms to open a new avenue in the development of multifunctional therapeutic nanosystems." | |
| The team is already working on designs that would allow to impart targeting ability for various malignant tumors and they have recently focused on treatments for breast cancer, brain cancer and liver cancer. Another challenge is to get a high enough drug payload delivered to the target sites. | |
| The researchers have reported their findings in the October 6, 2009 online edition of Advanced Functional Materials ("A Multifunctional Nanodevice Capable of Imaging, Magnetically Controlling, and In Situ Monitoring Drug Release"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
