| Posted: Feb 06, 2007 | |
Modeling the molecular force generation in cells |
|
| (Nanowerk Spotlight) In much the same way that each of our bodies depends on bones for mechanical integrity and strength, each cell within our bodies is governed mechanically by a skeleton of composite materials including protein polymers (such as actin filaments) and motor proteins (myosin), called the cytoskeleton. | |
| Actin and myosin are key components in muscle contraction and cell motility. The cytoskeleton is an active material that maintains cell shape, enables some cell motion, and plays important roles in both intra-cellular transport and cellular division. | |
| The cytoskeletal system is not at thermodynamic equilibrium and this non-equilibrium drives motor proteins that are the force generators in cells. Analogous to how our bones are held and moved by muscles, the cytoskeleton is activated by these molecular motors, which are nanometer-sized force-generating enzymes. | |
| In an ongoing effort to design and create a simplified, bottom-up model of the cytoskeleton, researchers now have designed and assembled a biomolecular model system capable of mechanical activity similar to that of living cells. | |
| This work could serve as a starting point for exploring both model systems and cells in quantitative detail, with the aim of uncovering the physical principles underlying the active regulation of the complex mechanical functions of cells. It could also provide new fundamental insights and offer new design principles for materials science. | |
 |
|
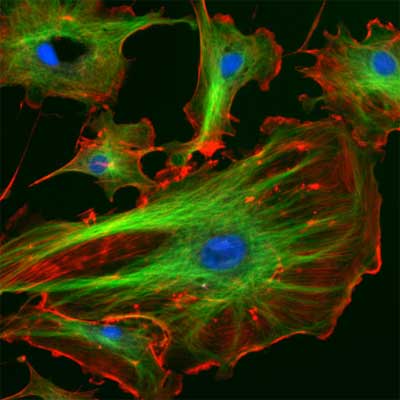
| The eukaryotic cytoskeleton. Actin filaments are shown in red, microtubules in green, and the nuclei are in blue. (Image: Wikimedia Commons) | |
| "We developed a simplified model of the active cytoskeleton by mixing actin with a crosslinker and myosin" Dr. Fred MacKintosh explains to Nanowerk. "This non-equilibrium (i.e. energy-consuming) material exhibits local contractions, similar to how they occur in most living cells. Tension generated by contraction can also stiffen the network up to 100-fold, in accordance with theory." | |
| While there have been many prior studies of in vitro/model networks created from the filamentous proteins or biopolymers that make up the cytoskeleton, these did not include the essential active ingredient of molecular motors that generate internal forces within the network. | |
| MacKintosh, who runs the Theory of Soft Matter and Complex Systems group at Vrije Universiteit Amsterdam in The Netherlands, is motivated by his group's theoretical efforts to develop quantitative models for cytoskeletal networks. In this recent work, in collaboration with Dr. Daisuke Mizuno, Dr. Catherine Tardin and Dr. Christoph Schmidt (at the University of Göttingen in Germany), the researchers present a quantitative theoretical model connecting the large-scale properties of the cytoskeletal actin-myosin material to molecular force generation. | |
| Besides modeling the molecular force generation, the second major innovation in this work was the ability to directly and simultaneously measure both the mechanical response (by active microrheology) and the fluctuations (by passive microrheology), thereby allowing them to test the fundamentally non-equilibrium nature of the system. | |
 |
|
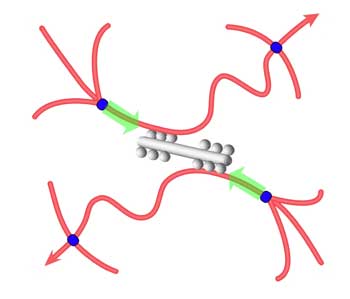
| Schematic illustration of tension development in actin filaments (red). Myosin minifilaments (gray) cause network contraction between cross-links (blue). (Image: Dr. MacKintosh) | |
| The findings demonstrate that a remarkably simple system, with just three components (myosin, actin and ATP), can reproduce key phenomena also observed in far more complex living cells. More generally, the results demonstrate an adaptive/active material that can tune its own mechanical properties. | |
| "This really opens the way to construct more complex bottom-up models of cellular structures and activity, which can then be quantitatively correlated with measurements in cells to elucidate the fundamentals of cell mechanics" says MacKintosh. | |
| He points out that one of the surprising findings of the work was the nearly 100-fold stiffening that occurred when the molecular motors became active. This opens interesting aspects for the design of "smart materials" with tunable mechanical properties. | |
| This system also represents a sort of micron-scale, isotropic muscle that can pull on its environment, much as when a cell crawls. | |
| "One of our main interests, and I think of others following this work, will be to create more complex models of the cytoskeleton and try to mimic and duplicate the activity of real cells" says MacKintosh. "While activity of this kind is measurable in cells, it is very hard to know what is responsible for it. Direct correlation with bottom-up constructs represents and exciting complementary approach to traditional knock-outs in cell biology, in which specific components are deactivated by genetics, for instance." | |
| These findings have been reported in the January 19, 2007 issue of Science (Nonequilibrium Mechanics of Active Cytoskeletal Networks). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
