| Posted: Dec 23, 2009 | |
DNA nanomachines that can be turned on and off with a flip of a switch |
|
| (Nanowerk Spotlight) Nanotechnology researchers already are constructing nanoscale machines and devices that can perform more and more complicated tasks. Interesting applications are being developed in a wide variety sciences ranging from basic research to pharmacology and medical treatment. One exciting application of nanomachines is their utilization as nanodrug delivery systems, which can be targeted to different tissues and can perform various tasks ranging from time-controlled drug release to gene therapy and cancer treatment. One challenge in designing these nanomachines is being able to establish how well they work and optimize their performance. This is where single molecule techniques will play an important role. | |
| With advances in nanotechnologies, it is possible to construct simple nanomachines that can perform simple functions such as opening and closing of a DNA device (e.g. DNA tweezers, see for instance "The gripping potential of DNA nanotechnology" or DNA switches, see "Synthetic DNA nanomachines go to work inside living cells"), small rotational and translational motors and energy transfer cascades. Using single-molecule techniques (such as the 'nanodumbbell' strategy we just reported), researchers can watch individual nanomachines working and determine the functionality of their design. | |
| Researchers in Germany now have incorporated optical addressability to these nanomachines. Hence, they can optically detect and eventually control the state of the nanodevice. | |
| "Much work has been invested in finding ways to self-assemble nanomachines. DNA, which can be programmed through its sequence, provides an excellent platform for forming DNA machines" Don C. Lamb, a professor in the Department of Chemistry and Biochemistry at the Ludwig-Maximilians-University of Munich, tells Nanowerk. "In previous work, we showed that DNA could be used as a scaffold for binding proteins and synthetic molecules and well known positions. Here, we have added the photoaddressability to turn FRET on and off between fluorophores mounted on the DNA. Hence, in a bottom-up approach, we have designed a self-assembling construct that provides information on the nanoscopic environment that is easily accessible and controllable from the macroscopic world." | |
| Specifically, Lamb and his team used the reversible photoactivation capabilities of the fluorescent protein Dronpa to switch Förster Resonance Energy Transfer (FRET) on and off between Dronpa and a synthetic fluorophore. | |
 |
|
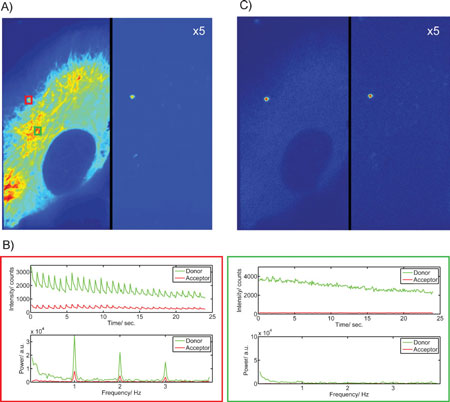
| Live-cell imaging experiments with a photoswitchable nanodevice. HeLa cells were stably transfected with eGFP-tubulin and incubated for 2 h with 300-nm polystyrol beads coated with streptavidin and the Dronpa–Atto647N–DNA constructs. The excitation is alternated with 2 frames of 405-nm excitation followed by 8 frames of 488-nm excitation and data were collected at a frame rate of 10 Hz or one cycle per second. A) The average fluorescence intensity over two 2 cycles (without 405-nm excitation), showing the eGFP and Dronpa in the left channel and the FRET signal in the right channel (multiplied by a factor of 5). B) The fluorescence intensity and power spectrum calculated from a 3x3 pixel region shown during the course of the movie taken from two different regions of the cell highlighted in green (upper panel) and red (lower panel) in panel (A). For clarity, the DC component has been excluded from the power spectrum plots. C) A filtered image showing the total power density at the switching frequency and higher harmonics calculated for each pixel. The improved contrast is clearly observable. (Reprinted with permission from Wiley-VCH Verlag) | |
| FRET is a phenomena that occurs when two molecules in very close proximity (∼10 nm) where, upon excitation of a fluorophore, energy is transferred from the higher energy molecule to the lower energy molecule. | |
| Lamb explains that, by modulating the fluorescence repetitively, they could apply lock-in detection, making it possible to detect our device within a background of GFP fluorescence, which has a very similar optical emission spectrum. | |
| Reporting their findings in a recent issue of Small ("Single-Molecule Investigations of a Photoswitchable Nanodevice"), the team has combined two key elements in nanobiotechnology – minute structural control and robust communication – in the environment of living cells. | |
| Using the photoswitching properties of this construct or similar devices as nanosenors, researchers can investigate the characteristics of the intracellular environment. The interaction between proteins and DNA can be visualized using FRET with strategically designed DNA probes. The lock-in detection can be used for visualizing biomolecules in live-cells or tissue with a high auto-fluorescing background. | |
| "For example" says Lamb, "when investigating virus entry using single-virus tracing, viruses labeled with complexes similar to what we have designed here can be distinguish from a fluorescence background via their photoswitching properties even when they are not distinguishable in the fluorescence intensity image." | |
| The team is currently implementing a variation of this technique to investigate conformational changes in viruses, where the desired signal would be too small to detect with traditional methods. Thereby, they hope to gain information over important steps in the viral life-cycle and investigate new potential pathways for treatment of viral diseases. | |
| Lamb points out that the ability to investigate a single device in action on the nanoscale provides essential information as to the strengths and weaknesses of the devices. | |
| "Optical lock-in methods will allow the detection of these nanodevices even in systems with a high backgrounds, such as experiments with drug delivery systems in tissue or in living systems," he says. "Optical switchability of nanodevices will provides a new element of control which will allow for the precise activation the these devices. Thus, in the future, complex nanomachines can be turned on and off with a flip of a switch." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
