| Posted: Jan 13, 2010 | |
Trying to understand the interaction of nanoparticles with blood |
|
| (Nanowerk Spotlight) Emerging nanotechnology applications in the fields of medicine and biology often involve the use of nanoparticles for probing biological processes and structures or for constructing sophisticated nanoscale drug delivery mechanisms. Nanoparticles are already being used with dramatic success in biomedical applications. However, relatively little is known about the potential biological risks from these nanoparticle applications inside the body. Consequently, a vast array of nanoparticles is being introduced into the environment as part of the nanotechnology revolution without regard to the potential toxic nature of these materials. There is no question that these are biologically active materials and it has become an extremely pressing problem to develop physical methods for screening for toxic responses of the promising new materials and for optimizing the medical efficacy. | |
| The identity of nanoparticles in a biological medium, in terms of their interaction with that medium, is largely determined by the proteins that dress the particles. Since many of the toxic and therapeutic uses of nanoparticles involve the introduction of nanoparticles into the bloodstream of humans and other animals, it is particularly important to know how nanoparticles interact with blood proteins. | |
| Although researchers have known that proteins adsorb onto nanoparticles and effect their interaction with the biological environment – such as whether the particles are taken up by cells – they have been lacking a systematic study of how particle size influences the formation of these protein layers. Until now. | |
| New research performed in the Polymers Division at the National Institute of Standards and Technology (NIST) directly addresses this issue and explores the effects of nanoparticle size (5nm to 100nm) and a whole range of important blood proteins. The main findings are that the thickness of the protein layer, the binding constant for the protein-nanoparticle interaction and degree of cooperatively in the protein-nanoparticle binding process all tend to increase with nanoparticle size, the effect saturating when the nanoparticles reach a size on the order of 60-80 nm. | |
| "Regardless of the blood protein considered, we found a general propensity of the protein-dressed particles to aggregate" Silvia H. De Paoli Lacerda and Jack F. Douglas tell Nanowerk. | |
| Lacerda is a researcher at the Center for Biological Evaluation and Research, Food and Drug Administration and Douglas is a NIST Fellow at NIST's Polymer Division. The two of them led a team of collaborators from the Universities of Maryland and Akton that focused on gold nanoparticles as the natural starting point for understanding nanoparticle-protein interactions. Gold nanoparticles are widely used in nanobiomedical research and, accordingly, the relevant literature is correspondingly vast and growing exponentially. | |
| In order to characterize various aspects of the protein-nanoparticle interaction, the team used a wide range of photophysical techniques, such as absorbance, fluorescence quenching, dynamic light scattering, circular dichroism (CD), and electron microscopy (EM). | |
 |
|
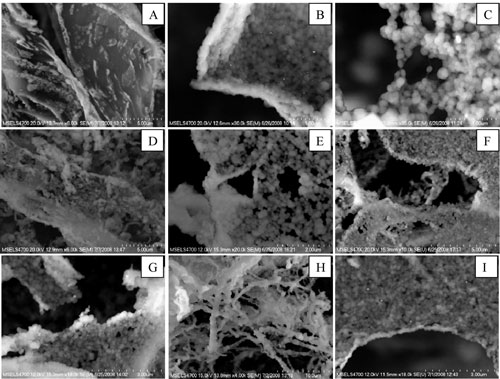
| Field-emission scanning electron microscopy (FESEM) images of clustered nanoparticle states resulting from different nanoparticle-protein combinations. (A) HSA, (B) HSA with 20 nm diameter gold nanoparticles, (C) HSA with 80 nm diameter gold nanoparticles, (D) fibrinogen, (E) fibrinogen with 20 nm diameter gold nanoparticles, (F) fibrinogen with 80 nm diameter gold nanoparticles, (G) histone (H3) with 80 nm diameter gold nanoparticle, (H) insulin with 80 nm diameter gold nanoparticle, (I) γ-globulin with 80 nm diameter gold nanoparticles. Images presented are representative of at least 20 different regions in each sample. (Reprinted with permission from the American Chemical Society) | |
| "These measurement methods allow for the determination of a number of basic properties" explain Lacerda and Douglas: "The binding (or association) constant of the proteins with gold nanoparticles, changes in protein conformation upon adsorption, nanoparticle size, nanoparticle aggregation, and thickness of the protein layer on the nanoparticle. Specifically, fluorescence quenching measurements give information about the protein-particle binding kinetics and equilibrium and protein conformational change, and absorbance and EM give information about the sizes of the bare and coated particles; CD informs about changes in protein structure upon binding, and dynamic light scattering and EM provide information about the formation and size of particle aggregates." | |
| By combining the use of these methods, the researchers were able to gain a general perspective of how nanoparticle size affects the nature of protein binding and the extent to which these effects are protein-specific. | |
| The researchers reported their findings in the December 18, 2009 online issue of ACS Nano ("Interaction of Gold Nanoparticles with Common Human Blood Proteins"). | |
| "Our choice of blood proteins in our study was motivated by clinical studies showing the increased incidence of heart attack in the case of combustion-derived nanoparticles introduced into the bloodstream through inhalation and by the increasing use of gold nanoparticles in biological imaging and cancer and other drug therapies," says Douglas. "It is an obvious scientific question and a matter of practical medical significance to understand how nanoparticles interact with blood proteins and we were simply trying to address this basic need. Perhaps as important as our work itself on these particular proteins and nanoparticles is the methodology to quantify these nanoparticle-protein interactions which can be applied to many types of nanoparticles and nanoparticles having different functionalities and shapes." | |
| The researchers note that there is a large number of problems related to the biological activity of nanoparticles that require serious scientific consideration and the development of new measurement techniques. | |
| "A serious complication of complex biological materials such as blood is that it contains literally thousands of blood proteins, a variety of cells, etc. and that the relative concentration of these species varies by orders of magnitude," explains Lacerda. "The adsorbed layer on the nanoparticles then evolves in time as species of higher affinity displace species adsorbed initially due to their higher initial availability due to their higher concentration. The study of the displacement process between different nanoparticles is then a crucial matter for investigation and already some preliminary studies of this effect are available. This is a very difficult problem for quantitative study since the protein concentrations are specific to an individual and his state of health." | |
| Another basic problem is that nanoparticles often tend to aggregate even without the proteins due to their high interfacial interactions and it is difficult to assess protein-particle interactions when the nanoparticles are already in a non-equilibrium flocculated state. | |
| Lacerda and Douglas point out that scientists also need to study how adsorption of proteins on nanoparticles alters the propensity of the dressed particles to associate onto biological and non-biological interfaces as an extension of the worked described above. This is a crucial problem with regard to how nanoparticles interact with biological systems. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
