| Posted: Feb 01, 2010 | |
Plasmonic nanobubbles combine diagnosis and treatment in one theranostic method |
|
| (Nanowerk Spotlight) Multifunctional nanoparticles are at the core of a growing field called theranostics that develops technologies physicians can use to diagnose and treat diseases in a single procedure. The major promise of theranostics is to bring together key stages of a medical treatment, such as the diagnosis and therapy, and thus to make a treatment shorter, safer and more efficient. | |
| "Theranostic approaches require adequate tools with a high multi-functionality and selectivity" Dmitri Lapotko explains to Nanowerk. "The initial phase of the development of theranostics has already revealed the two general challenges: lack of multifunctional methods and agents, and the lack of selectivity and specificity of available agents – that ultimately requires cell and molecular levels." | |
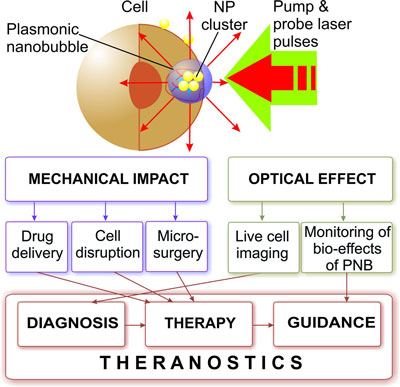
| Lapotko and his colleagues at the Joint US-Belarussian lab for fundamental and biomedical nanophotonics at Rice University, in collaboration with researchers at the A.V.Lykov Heat and Mass Transfer Insitute (Belarus) and M.D.Anderson Cancer Center (Houston, TX), have developed a novel method based on gold nanoparticle-generated transient photothermal vapor nanobubbles, a structure they refer to as plasmonic nanobubbles (PNB). These dynamically tuned intracellular plasmonic nanobubbles are well suited for cell theranostics since they combine diagnosis (through optical scattering), therapy (through mechanical, nonthermal and selective damage of target cells) and optical guidance of the therapy into one fast process. | |
 |
|
| Mechanism and medical applications of plasmonic nanobubbles. (Illustration: Dr. Lapotko, Rice University) | |
| In a paper in the January 25, 2010 online issue of Nanotechnology ("Tunable plasmonic nanobubbles for cell theranostics"), Lapotko and his team have now presented the first and laboratory stage proof of the principle for theranostics with plasmonic nanobubbles. | |
| "We hypothesized that a combination of the photothermal properties of plasmonic nanoparticles with those of transient vapor bubbles may be a key solution of some of the above-mentioned problems with theranostic approaches," says Lapotko. "We achieved this through the development of a tunable nanoscale theranostic probe that is not a nanoparticle but a nanoparticle-generated event – the plasmonic nanobubble, which combines high optical brightness with localized mechanical impact. The PNB is a system that results from the interaction of optical radiation with a nanoparticle and its environment." | |
| In their recent work, the Rice team have studied the optical generation and detection of PNBs around gold nanoparticles in individual living cells, with the focus on tuning the nanobubble properties in one cell and evaluating the multi-functionality of the PNB. | |
| Compared to nanoparticle-based hypothermia, diagnosis and delivery methods, plasmonic nanobubbles act at molecular and cell levels, thus providing high localization of the impact. This nanoscale approach has three clear advantages: 1) improved sensitivity, specificity of diagnostic applications; 2) improved selectivity and guidance of therapeutic applications; 3) combined diagnosis, therapy and therapy guidance in a single process at the cellular level. | |
| Lapotko explains that the optical and mechanical properties of a plasmonic nanobubble depend on its diameter – which is tunable in a range of 50 nm to 50 µm – and lifetime – which is tunable in a range of 10 ns to 10 µs. The short lifetime of PNBs makes them highly transient phenomena that exist on demand. | |
| here is how the system works: For target-specific generation of the PNBs clusters of relatively safe gold nanoparticles, conjugated to diagnosis-specific antibodies, are selectively aggregated into clusters around molecular targets in cancer cells (through the mechanisms of antibody?antigen interaction and endocytosis). | |
| "We realized the remote (optical) and non-invasive activation and sensing of PNBs around such intracellular cluster in individual living cells with free laser beams" says Lapotko. "When activated by a laser pulse, an intracellular plasmonic nanoparticle acts a heat source and generates a transient PNB in the surrounding medium. PNBs of nanometer-scale size and nanosecond-scale duration act as diagnostic probes by scattering light from the probe laser. Larger micrometer-scale PNBs provide a localized therapeutic action through a mechanical (non-thermal) impact due to their rapid expansion and collapse, thus disrupting the cell membrane." | |
| In summary, these plasmonic nanobubbles promise a safe and highly effective on-demand therapeutic nanoscale platform – PNBs can be selectively generated in specific cells, do not use chemicals, and rely only on nanoscale phenomena of light and heat that are natural for all living systems. | |
| "We believe that in the future, PNBs can provide a universal platform for basic biomedical research, diagnosis and therapy" says Lapotko. "Potential applications of PNBs include 1) high-sensitivity non-invasive imaging, 2) controlled release, transfection and intracellular delivery, and 3) selective and guided cell and tissue damage. Although aimed at cancer, our methods are universal and can be applied to other pathological conditions since the plasmonic nanobubbles can be used at the molecular, cellular and tissue levels." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
