| Posted: Mar 19, 2010 | |
Nanotechnology recycles environmental energy waste into hydrogen fuel |
|
| (Nanowerk Spotlight) Materials that can produce electricity are at the core of piezoelectric research and the vision of self-powering machines and devices (see for instance: "Electricity-generating silicone implants could power electronic devices"). With the emergence of nanotechnology and the use of nanomaterials, the field of piezoelectrics and nanopiezotronics has experienced a lot of new and interesting research efforts. A recent study, for instance, has demonstrated that the small vibrational energy waste generated in the environment from noise, wind power, running water, or water wave action can be scavenged or harvested as a driving force for direct water splitting. | |
| "We propose a new piezoelectrochemical mechanism for the direct conversion of mechanical energy to chemical energy and subsequently the splitting of water into hydrogen and oxygen" Huifang Xu tells Nanowerk. "We have demonstrated our technique by growing nanocrystals of two common crystals, zinc oxide and barium titanate, and placing them in water. When pulsed with ultrasonic vibrations, the nanofibers flexed and catalyzed a chemical reaction to split the water molecules into hydrogen and oxygen." | |
| Xu, an assistant professor in Mineral Science, Nano-geoscience, and Electron Microscopy at the University of Wisconsin-Madison, explains when the fibers bend, asymmetries in their crystal structures generate positive and negative charges and create an electrical potential. | |
| "This phenomenon, called the piezoelectric effect, has been well known in certain crystals for more than a century and it is the driving force behind quartz clocks and other applications" he says. "However, while the bulk materials are brittle, at the nanoscale they are flexible – it's like the difference between fiberglass and a pane of glass." | |
 |
|
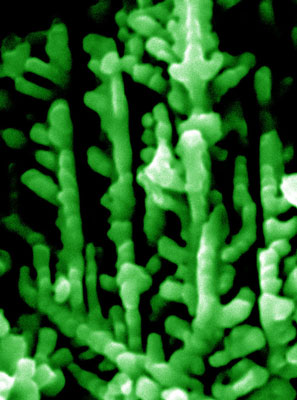
| A scanning electron microscopic image of a tree-like micro-dendrite with piezoelectrochemical property for scavenging mechanical energy wastes from environment. When the branches of the dendrite are bent, induced electrical potential will build up on their surfaces for splitting water and catalyzing other chemical reactions. (Image: Dr. Huifang Xu, University of Wisconsin-Madison) | |
| Xu and his team demonstrated the piezoelectrochemical effect of their nanofibers by placing them in water and applying ultrasonic vibrations to them. Under the impact of the ultrasonic vibrations, the nanofibers flexed and catalyzed a chemical reaction to split the water molecules into hydrogen and oxygen. | |
| "The physics and chemistry of generating hydrogen and oxygen gases from pure water arise from the combination of piezoelectric properties of the nanofibers and the redox reaction of water"explains Xu. "The piezoelectricity of each material arises from the lack of inversion symmetry in their crystal structures. Any deformation or strain acting on the material will cause a nonzero dipole moment in the crystal lattice. Consequently, a strain-induced charge potential is produced on the surface of the material." | |
| The strain-induced electric potential formed on the surface of the nanofiber in wet conditions (i.e., in pure water) is then available for the reduction and oxidation reaction via charge transfer to water molecules adsorbed on the surface. However, notes Xu, the developed potential must be greater than the standard redox potential of water (1.23 eV) to make electrons available to initiate the redox reaction in such a setup. | |
| "Smaller fibers, especially on the nanoscale, bend more easily than larger crystals and therefore also produce electric charges easily. And because we can tune the fiber and plate sizes, we can use even small amounts of mechanical noise – like a vibration or water flowing – to bend the fibers and plates. With this kind of technology, we can scavenge energy waste and convert it into useful chemical energy." | |
| Xu points out that the principle of the piezoelectrochemical effect using these fibers could be a very important step forward in nanotechnology that recycles the energy wastes from the environment into precious alternative chemical energy. This work will open a new field of study on hydrogen generation, redox reactions, and energy recycling. | |
| "We have limited areas to collect large energy differences, like a waterfall or a big dam," he says. "But we have lots of places with small energies. If we can harvest that energy, it would be tremendous." | |
| However, he cautions that the team still needs to better understand important steps that are limiting the overall reaction, and develop a scale-up device/system for converting mechanical energy into hydrogen energy. | |
| "One of the challenges we need to overcome is the development of effective and durable fibers or plates with difference sizes for harvesting different level of mechanical energy wastes" says Xu. | |
| The University of Wisconsin-Madison team has reported in the March 2, 2010 online issue of Journal of Physical Chemistry Letters ("Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
