| Posted: Mar 23, 2007 | |
Nanotechnology probes and biosensors |
|
| (Nanowerk Spotlight) Biosensors, which incorporate biological probes coupled to a transducer, have been developed during the last two decades for environmental, industrial, and biomedical diagnostics. The application of nanotechnology to biosensor design and fabrication promises to revolutionize diagnostics and therapy at the molecular and cellular level. The convergence of nanotechnology, biology, and photonics opens the possibility of detecting and manipulating atoms and molecules using a new class of fiberoptic biosensing and imaging nanodevices. These nanoprobes and nanosensors have the potential for a wide variety of medical uses at the cellular level. The potential for monitoring in vivo biological processes within single living cells, e.g. the capacity to sense individual chemical species in specific locations within a cell, will greatly improve our understanding of cellular function, thereby revolutionizing cell biology. Existing nanoprobes have already demonstrated the capability of performing biologically relevant measurements inside single living cells. | |
| A fiberoptic nanosensor basically is a nanoscale probe that consists of a biologically or chemically sensitive layer that is covalently attached to an optical transducer. Biological sensing elements can be either a biological molecular species (e.g., an antibody, an enzyme, a protein, or a nucleic acid) or a living biological system (eg, cells, tissue, or whole organisms) that uses a biochemical mechanism for recognition. In the case of a receptor-based nanosensor, an interaction between the immobilized receptor and its substrate – the molecule it binds to – produces a perturbation that the optical transducer converts to an electrical signal via laser-induced fluorescence. | |
| Dr. Tuan Vo-Dinh, Professor of Biomedical Engineering and Chemistry and Director of the Fitzpatrick Institute for Photonics at Duke University's Pratt School of Engineering, has devoted extensive research and development to the development of a variety of fiberoptic chemical nanosensors and nanobiosensors. | |
| "Preparing fiberoptic nanosensors is fairly straightforward if one has the right tool and good practice" Vo-Dinh explains to Nanowerk. "Using the so-called "heat and pull" method, a micron-size diameter silica optical fiber is placed in a commercially available puller that heats the fiber using a carbon dioxide laser and then pulls the fiber to the desired thickness, usually between 20 an 100 nanometers in diameter. The pulled fiber is then cut in half, yielding two nanoscale fiber tips. Vapor deposition is then used to deposit a thin layer of silver, aluminum or gold on the side walls of the tip, followed by a two-step chemical treatment of the tip that provides covalent attachment points for the biosensor molecules." | |
 |
|
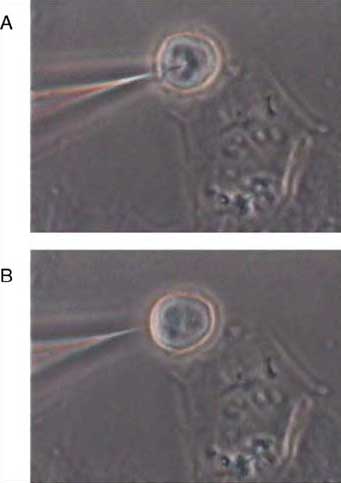
| Digital image of single-cell sensing. The bottom image B, shows an optical nanosensor before insertion and a single cell, whereas the top image A, shows the optical nanosensor inserted into the cell. (Reprinted with permission from Elsevier) | |
| The need for fast and specific assays has induced researchers to explore alternative optical detection technologies for diagnostic applications. In addition to the nanobisensor technology, Vo-Dinh and co-workers have developed a new type of nanoprobes using Raman and SERS (Surface Enhanced Raman Spectroscopy) detection. | |
| "Due to the inherently small Raman cross section, Raman spectroscopy has not been widely used in the past for trace analysis" says Vo-Dinh. "Nevertheless, there has been a renewed interest in Raman techniques in the past two decades as a result of the discovery of the SERS effect." | |
| In SERS the Raman effect is found to be greatly enhanced when it is close to a rough metal surface consisting of gold or silver nanoparticles, due to surface plasmon resonance. In recent years it has been demonstrated that single-molecule detection with SERS is possible. | |
| Vo-Dinh and his collaborators have demonstrated the feasibility of SERS imaging of cell surface receptors using SERS nanoprobes. In a paper, titled "Nanoprobes and nanobiosensors for monitoring and imaging individual living cells" they describe the plasmonics technology using SERS nanoprobes to deliver chemically significant images with both spatial and spectral information of chemical components in cellular components. | |
| Vo-Dinh points out that a significant advantage of the nanobiosensors for cell monitoring is the minimal invasiveness of the technique. Optical nanobiosensors thus hold promise for dynamic analyses of proteins in biochemical pathways within single living cells. | |
| "These optical nanoprobes provide a new method in cell based assays offering highly miniaturized nanoscale devices that make cell-based analysis accessible at the single-cell level" says Vo-Dinh. "Future applications of optical nanoprobes could include multianalyte detection and analysis of protein-protein interactions and similar analyses of other proteins involved in cellular biochemical pathways." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
