| Posted: Jul 12, 2010 | |
Nanobiocomposite antimicrobial surface coatings based on carbon nanotubes |
|
| (Nanowerk Spotlight) Life-threatening infectious diseases caused by antibiotic-resistant pathogens have been of great concern in both community and hospital settings. This increasing emergence of antibiotic-resistant strains of pathogens has necessitated the development of new antimicrobial surfaces and coatings. As antimicrobial surfaces have become popular in such areas as consumer products, public spaces such as schools and offices, and public transportation, the market for these coatings has quickly grown into a market worth hundreds of million of dollars. | |
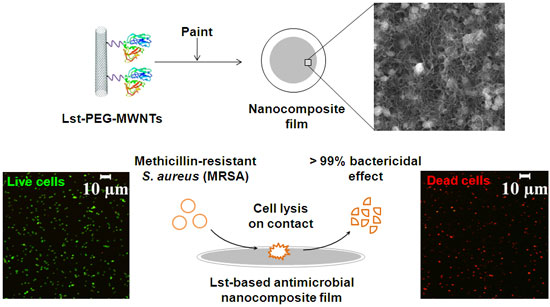
| In a previous Nanotechnology Spotlight we have reported on how researchers have developed an effective antimicrobial coating by combining carbon nanotubes and natural materials. New work, by a team from Rensselaer Polytechnic Institute (RPI), has now combined the antimicrobial property of a cell lytic enzyme (lysostaphin) and the excellent properties of carbon nanotubes as an immobilization support in preparing nanocomposite paints that are highly effective against antibiotic-resistant strains of Staphylococcus aureus – methicillin-resistant Staphylococcus aureus (MRSA). | |
| "Using our approach, which exploits naturally evolved and species-specific cell lytic enzymes, it is possible to develop paints and coatings that are effective against specific pathogens," Ravi S. Kane, the P. K. Lashmet Professor of Chemical and Biological Engineering at RPI and co-principal investigator of the research team, tells Nanowerk. "While the use of traditional antibiotics to kill such pathogens has resulted in an increasing number of resistant microbes, cell lytic enzymes have been shown previously to be far more difficult for microbes to overcome and become resistant. This is particularly the case with lysostaphin against S. aureus," adds Jonathan S. Dordick, the Howard P. Isermann Professor of Chemical and Biological Engineering and Director of RPI's Center for Biotechnology & Interdisciplinary Studies, and co-principal investigator of the research team. | |
| The paints developed by the Dordick-Kane-led team were capable of killing staphylococci, including MRSA, on contact and without release of antimicrobial agents. These results suggest that such paint composite films with embedded antimicrobial lysostaphin-nanotube conjugates may be effective in preventing the risk of staphylococci-specific infection and biofouling of common surfaces. | |
| "We believe that the approach is not limited to lysostaphin alone, and it can be extended to other cell lytic enzymes to obtain a surface with broader activity against various pathogenic bacteria" says Kane. | |
 |
|
| The fabricated nanocomposites contained different conjugate formulations and enzyme loadings. These enzyme-based composites were highly efficient in killing MRSA (>99% within 2 hours) without release of the enzyme into solution. Additionally, these films were reusable and stable under dry storage conditions for a month. (Image: Ravi Pangule, Rensselaer Polytechnic Institute) | |
| Reporting their findings in the July 6, 2010 online issue of ACS Nano ("Antistaphylococcal Nanocomposite Films Based on Enzyme Nanotube Conjugates"), the team notes that their enzyme-based coatings were found to be reusable and stable over a month. | |
| "The molecular-level curvature of carbon nanotubes stabilizes a wide range of enzymes, while the high surface area-to-volume ratio enables high catalytic loading without diffusional limitations" explains Dordick. "In addition, the high aspect ratio of the carbon nanotubes leads to efficient entanglement within the solid matrix, thereby allowing enzymes to be retained." | |
| For their experiments, the team attached lysostaphin to multiwalled carbon nanotubes (MWCNT). They found that the bactericidal activity of the enzyme could be improved by providing a flexible spacer between lysostaphin and the MWCNT surface. Therefore, they covalently attached lysostaphin to the nanotube surface through a short poly(ethylene glycol) (dPEG12 or PEG) linker. | |
| "The PEG linker showed a 2-fold increase in the bactericidal activity during short exposure times (about 1 minute) compared to lysostaphin-MWCNT, and killed ∼99% of S. aureus cells in approximately 10 minutes" says Ravi Pangule, a Ph.D. student and member of the research team. | |
| To find out if lysostaphin-based conjugates are effective against other bacteria, the team performed similar cell-based assays with microbial suspensions of Staphylococcus epidermidis, Escherichia coli (E. coli), and Bacillus cereus (B. cereus). The results were consistent with the known activity of lysostaphin. For instance, the conjugates were effective against S. epidermis but were ineffective against nonstaphylococcal cells, E. coli and B. cereus. | |
| "This indicates that the enzyme-MWCNT conjugates do not possess indiscriminate cytotoxicity" Kane points out. "The aggregate of our results indicates that lysostaphin-based conjugates show effective and selective bactericidal activity toward staphylococci." | |
| On a broader scale, such nanobiotechnology-based approaches can be used in developing enzyme-based coatings for antifouling applications, and for decontamination of various chemical and biological warfare agents. | |
| The team cautions, however, that one of the challenges in preparing functional nanobiocomposites is to reduce the inherent loss of enzymatic activity on immobilization. To address this concern, fundamental studies involving the impact of the nanoscale environment on protein structure and stability are needed, and the team is doing just that. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
