| Posted: Jul 16, 2010 | |
New solar-powered process removes carbon dioxide from the air and stores it as solid carbon |
|
| (Nanowerk Spotlight) The alarming rise of carbon dioxide in the atmosphere has led to numerous proposals on how to capture and store CO2 in order to mitigate the damaging emissions from fossil fuels. Popular proposals, some already being tested on a large scale, involve carbon sequestration and subsequent storage in geological formations (geo-sequestration). Other ideas revolve around recycling captured carbon dioxide, for instance by converting it into hydrocarbons that can be re-used to make fuel or plastics. While these solutions would result in removing some carbon dioxide from the atmosphere, their disadvantages are that most of them are expensive, technologically challenging, or energy-intensive. | |
| Researchers have now presented the first experimental evidence of a new solar conversion process, combining electronic and chemical pathways, for carbon dioxide capture in what could become a revolutionary approach to remove and recycle CO2 from the atmosphere on a large scale. Rather than trying to sequester or hide away excess carbon dioxide, this new method allows it to be stored as solid carbon or converted in useful products ranging from plastics to synthetic jet fuel. | |
| "The STEP (Solar Thermal Electrochemical Photo) process proactively converts anthropogenic carbon dioxide generated in burning fossil fuels, as well as eliminates carbon dioxide emissions associated with the generation of metals and bleach," Stuart Licht, a professor in the Department of Chemistry and Solar Institute at George Washington University, explains to Nanowerk. "Our new STEP carbon capture process is the culmination of over 20 years of ongoing research, starting with developing solar energy to drive chemical, rather than only electronic, energy ("A description of energy conversion in photoelectrochemical solar cells"). In 2003, we set the theoretical basis that solar visible and solar thermal sunlight will provide a synergistic enhancement of solar energy conversion efficiency, and in 2009 the theoretical basis for STEP carbon capture ("STEP Generation of Energetic Molecules: A Solar Chemical Process to End Anthropogenic Global Warming")." | |
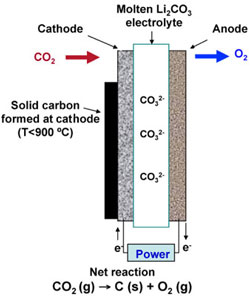 |
|
| Molten carbonate electrolysis system. (Images: Dr. Licht, George Washington University) | |
| The experimental verification that Licht and his collaborators have now published in the July 14, 2010 issue of The Journal of Physical Chemistry Letters ("A New Solar Carbon Capture Process: Solar Thermal Electrochemical Photo (STEP) Carbon Capture") is a major development. | |
| Licht explains that the STEP process is a synergy of solid-state and solar thermal processes, and is fundamentally capable of converting more solar energy than photovoltaic or solar thermal processes alone. | |
| "Here, CO2 is captured using a high temperature electrolysis cell powered by the full spectrum of sunlight in a single step. Solar thermal energy decreases the energy required for carbon capture, while visible sunlight generates electronic charge to drive the electrolysis. Carbon dioxide can be captured from 34% to over 50% solar energy efficiency – depending on the level of solar heat inclusion – as solid carbon and stored, or used as carbon monoxide to be available for a feedstock to synthesize – with STEP generated hydrogen – solar diesel fuel, synthetic jet fuel, or chemical production." | |
| As Licht points out, this research crosses over several fields. "Using the terminology of a chemical physics, rather than tune the semiconductor bandgap, we tune the electrochemical potential, to achieve unprecedented solar efficiencies of carbon capture. In the language of an electrochemist, we have resolved the challenge to convert stable carbon dioxide to useful energetic molecules, by isolating a medium (lithium carbonate), which supports effective thermodynamic (low energy) and kinetic (high power) electrolysis. Finally, in the language of a solar researcher, we convert sunlight to useful energy at unprecedented solar energy efficiencies through the synergy of using the complete solar spectrum." | |
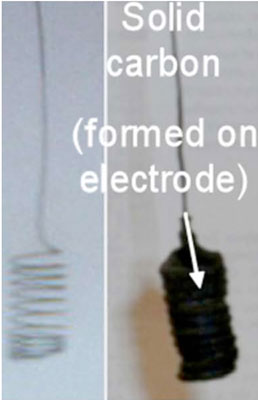 |
|
| Molten carbonate electrolysis system. (Image: Dr. Licht, George Washington University) | |
| Previously, solar cells disposed of solar thermal radiation as it was destructive to photovoltaics, and had diminished both output and stability. In contrast, STEP utilizes not only visible sunlight but also the usually detrimental thermal component of sunlight for the electrolytic formation of chemicals and thereby uses this extra energy to achieve solar conversion efficiencies higher than the best photovoltaics. | |
| In experiments, the team has demonstrated that a photovoltaic system, converting solar to electronic energy at 37.0% efficiency and 2.7 V, may be used to drive three electrolysis cells, each operating at 0.9 V, splitting CO2, and each generating a 1.3 V CO product. | |
| Licht and his team are extremely optimistic about the impact of the STEP technology: "This STEP process provides a path to lower atmospheric carbon dioxide to pre-industrial levels" he says. | |
| In addition to carbon capture, the researchers have already successfully applied the STEP process to using solar energy to efficiently generate hydrogen fuel from water (they will report this in an upcoming paper in The International Journal of Hydrogen Chemistry) and they are in the midst of applying the STEP process to a variety of useful energetic molecules ranging from metals to bleach at high solar efficiency from naturally occurring resources. | |
| According to Licht, challenges that remain with STEP include stability and cost of materials, activity of electrocatalysts, effective utilization of excess heat, batch versus continuous process for extracting solid carbon as a cathode product, and the systems engineering of spectrum-splitting concentrators to increase the availability of dichroic and dielectric beam splitters. | |
| "The next challenge is looking for support to scale-up STEP carbon capture from the laboratory to the outdoor test environment" says Licht. "A key part of the STEP process operates in similar, but reverse manner to MCFCs (molten carbonate fuel cells), which have already faced, and solved, the materials issues of operating in these unusual high temperature molten carbonate conditions." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
