| Posted: May 02, 2007 | |
Real-time single-molecule imaging of an entire chemical reaction |
|
| (Nanowerk Spotlight) It its more than 25 years of existence, Scanning Tunneling Microscopy (STM) has predominantly brought us extremely detailed images of matter at the molecular and atomic level. STM - not to be confused with the scanning electron microscope (SEM) - is a non-optical microscope that scans an electrical probe over a surface to be imaged to detect a weak electric current flowing between the tip and the surface. The STM allows scientists to visualize regions of high electron density and hence infer the position of individual atoms and molecules on the surface of a lattice. Researchers have now taken a further step by using STM to perform real-time single-molecule imaging of an entire chemical reaction. Many chemical reactions are catalyzed by metal complexes, and insight into their mechanisms is essential for the design of future catalysts. These new findings have demonstrated that the STM approach to studying chemical reactions in a dynamic environment can provide valuable information about reaction mechanisms and rates, as well as catalyst activity and stability. | |
| "A couple of years ago, we took up the idea of imaging all the steps of a chemical reaction in an STM" Dr. Hans Elemans tells Nanowerk. "Some examples of imaging chemical reactions have been described before, but only under extreme conditions (low temperature, ultrahigh vacuum), and we wanted to make it work in an environment familiar to chemists, i.e. the interface of a liquid and a solid. As such, we are now the first ever who succeeded in imaging a chemical reaction in such a medium." | |
 |
|
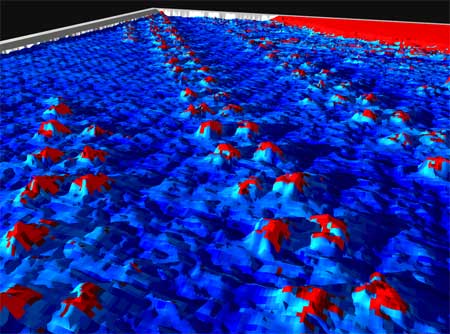
| 3D STM image of arrays of manganese porphyrin catalysts at an interface of a tetradecane liquid and a gold surface. | |
| Elemans, a member of the Nolte Group for Supramolecular Chemistry at Radboud University in Nijmegen, The Netherlands, together with members from his group and collaborators from the University of Sydney and the Institute for Molecules and Materials at Radboud University, are the first to image with STM a complete catalytic reaction at the interface of a liquid and a solid by following the catalyst molecules, when they are in action, at the single molecule level. | |
| The researchers used manganese porphyrins, dye molecules of which for example the naturally occurring magnesium analogues are involved in processes such as light-harvesting in plants, as the catalysts for their chemical reactions. In their experiments, manganese porphyrin was added to a liquid-cell STM set-up and the researchers were able to observe the formation of extended monolayer domains at the liquid–solid interface, with the porphyrins adsorbed face-on to the surface in regular patterns. | |
| "The reaction that we investigated led to several unexpected new insights" explains Elemans. "First, the gold surface used appeared to activate the adsorbed manganese porphyrin catalysts to react with molecular oxygen (O2). When the catalysts are in their dissolved state in a solution, this reaction does not occur. The second surprise was that each molecule O2 appeared to have a preference to oxidize two adjacent catalysts. This perfectly illustrates that STM is a unique technique, because it measures so locally i.e. at the single molecule level, that such a process can be identified. A final surprise was that the surface-bound catalysts were extremely stable. In case of similar reactions in solution, manganese porphyrin catalysts cluster together and oxidize themselves. At the surface, they remain well-separated from each other and many turnovers per single catalyst could be observed." | |
 |
|
| Photograph of the home-built Nijmegen Liquid Cell STM in which the catalysis experiments were carried out. (Image: Dr. Elemans) | |
| The researchers' motivation for this work was that they wanted to develop an additional technique to study a chemical reaction. Nearly all conventional techniques are ensemble techniques and measure the average behavior of millions of molecules at a certain time. STM measures the behavior of single molecules and as such it might be used in the future as an additional analytic technique to the conventional ones. | |
| Elemans points out that there are drawbacks to the new technique. One of the problems still is that STM is a slow technique. Scanning occurs in the order of seconds, and if processes that one wants to study are faster they might not be captured by the STM. | |
| "For that reason, the oxidation reaction chosen by us was known to be also very slow so we were sure that we were looking at real-time processes" he says. "However, it limits the use of this technique for studying many other, faster reactions. Solving the problem relies on the development of faster STM equipment, which is in fact currently ongoing." | |
| Being able to gain a highly detailed insight into chemical reaction mechanisms might one day enable chemical engineers to improve the reaction, and, on an industrial scale, maybe improve entire chemical synthesis and catalysis processes. For now, this is only a vision, though, but the possibilities are almost endless. | |
| "This is only the first chemical reaction investigated with STM at a liquid-solid interface, and there might be thousands of reactions more which can be investigated in a similar manner" says Elemans. "All of these might give new insights. Currently our group is investigating other types of reactions with similar porphyrin catalysts. A particular challenge is to study cascade reactions in such a way. These are reactions in which two or more different catalysts are bound to the surface, and in which the product of a catalytic reaction at a catalyst A is the starting material of the reaction at catalyst B. It is of great interest to see how such reactions are coupled in time and space, at the single molecule level." | |
| This work, titled "Real-time single-molecule imaging of oxidation catalysis at a liquid–solid interface", was published in the April 22, 2007 online edition of Nature Nanotechnology. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
