| Posted: Nov 05, 2010 | |||
Nanocatalysis: Applications in the chemical industry |
|||
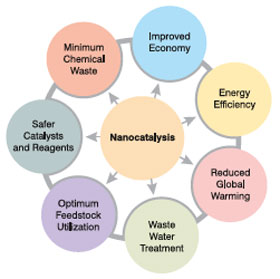
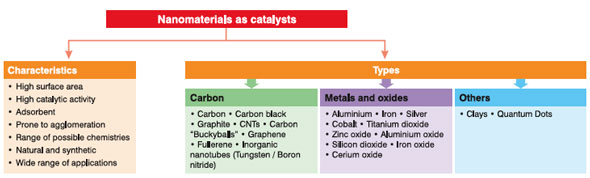
| (Nanowerk Spotlight) Nanocatalysis is a rapidly growing field which involves the use of nanomaterials as catalysts for a variety of homogeneous and heterogeneous catalysis applications. Heterogeneous catalysis represents one of the oldest commercial practices of nanoscience; nanoparticles of metals, semiconductors, oxides, and other compounds have been widely used for important chemical reactions. | |||
| Although surface science studies have contributed significantly to our fundamental understanding of catalysis, most commercial catalysts, are still produced by "mixing, shaking and baking" mixtures of multi-components; their nanoscale structures are not well controlled and the synthesis-structure-performance relationships are poorly understood. Due to their complex physico-chemical properties at the nanometer scale, even characterization of the various active sites of most commercial catalysts proves to be elusive. | |||
 |
|||
| A key objective of nanocatalysis research is to produce catalysts with 100% selectivity, extremely high activity, low energy consumption, and long lifetime. This can be achieved only by precisely controlling the size, shape, spatial distribution, surface composition and electronic structure, and thermal and chemical stability of the individual nanocomponents. In this article, the exciting opportunities of nanocatalysis in chemical and refining processes, as well as the challenges in developing nanostructured catalysts for industrial applications, are discussed. | |||
| The field of nanocatalysis (the use of nanoparticles to catalyze reactions) has undergone an explosive growth during the past decade, both in homogeneous and heterogeneous catalysis. Since nanoparticles have a large surface-to-volume ratio compared to bulk materials, they are attractive candidates for use as catalysts. | |||
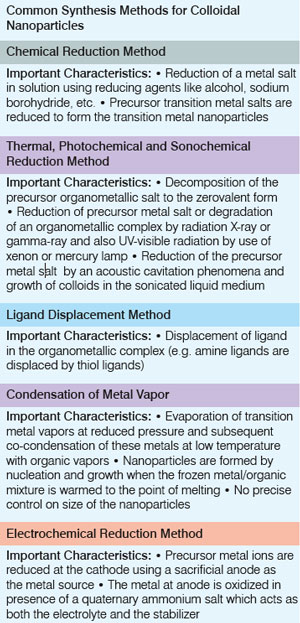
In homogeneous catalysis, transition metal nanoparticles in colloidal solutions are used as catalysts. In this type of catalysis, the colloidal transition metal nanoparticles are finely dispersed in an organic or aqueous solution, or a solvent mixture.
The different reduction methods that have been used to synthesize colloidal transition metal nanoparticles for homogeneous catalysis are summarized below. Chemical reduction of the precursor transition metal salt is the most widely used method of synthesizing transition metal nanocatalysts in colloidal solution. There are four other synthetic methods to prepare colloidal transition metal nanocatalysts that are not as commonly used. These synthetic methods include A good stabilizer is one that protects the nanoparticles during the catalytic process, but does not neutralize the surface of nanoparticles resulting in loss of catalytic activity. The choice of a stabilizer to be used for capping the nanoparticles is usually a balancing act between passivation of the nanoparticle surface and the fraction of available sites for catalysis, and also affects the size and shape of the nanoparticles formed. |
|||
| Heterogeneous metal nanocatalysts are prepared by adsorption of nanoparticles on to supports, which involves functionalization of supports to adsorb nanoparticles on to them and, fabrication of nanostructures on the supports by lithographic techniques. | |||
Benefits of nanocatalysts in the chemical industry
In view of the numerous potential benefits that can accrue through their use, nanostructured catalysts have been the subject of considerable research attention in recent times. Many applications and patents have also been realized adopting such nanostructured catalysts leading to significant process improvements as exemplified below. Important Applications of Nanocatalysis Sector: Biomass Application: Biomass gasification to produce high syn gas and biomass pyrolysis for production of bio-oil |
|||
| Process Improvements: • Novel Al2O3 supported NiO catalyst reduces tar yield significantly and increases tar removal efficiency to 99% • Significant increase in gas yield • Lighter fractions of H2 & CO are increased in the syn gas composition while heavier fractions of CH4 & CO are reduced, thus improving syn gas quality | |||
| Catalyst: Nano NiO catalyst supported on γ- Al2O3 microspheres of 3 mm size (Johnson Mathey Company, greater than 99% purity) | |||
| Application: Production of biodiesel from waste cooking oil | |||
| Process Improvements: • Esterification of fatty acids (FFAs) and transesterification of triglycerides to biodiesel in one pot • Solid acid nanocatalysis of Al0.9H0.3PW12O40 nanotubes with double acid sites yield 96% of biodiesel from waste cooking oil as compared to 42.6% with conventional H3PW12O40 catalyst Catalyst: Aluminium dodeca-tungsto-phosphate (Al0.9H0.3PW12O40) nanotubes as solid catalysts with surface area of 278 m2/g | |||
| Application: Green diesel production using Fischer-Tropsch Synthesis (FTS) | |||
| Process Improvements: • Improving the FTS technology for production of high molecular weight waxes, followed by their hydrocracking to generate liquid fuels • Improved efficiency of slurry and fixed-bed reactors, used in FTS from biosyngas • Produce long, linear-chain paraffin waxes in fixed bed & slurry FTS reactors | |||
| Catalyst: • Nano Fe and Co powders (10-50 nm) are used as FTS catalysts in slurry reactors, promoted by other metals like Mn, Cu & alkalis • Produced by thermal plasma chemical vapor deposition (TPCVD) and cluster spray techniques • Minimize liquid-solid diffusion resistance • Multi-walled carbon nanofilaments (MWCNF), produced by CO2 sequestration via dry reforming for gas-to-liquid FTS, with the iron carbide content rendering catalytic activity | |||
| Sector: Oil, Gas & Fossil Fuels | |||
| • Paraffin Dehydrogenation • Naphtha Reforming • Selective Hydrogenation • Hydrodesufurization | |||
| Application: Improved economic catalytic combustion of JP-10 aviation fuel using hydrocarbon fuel soluble nano catalyst | |||
| Process Improvements: • 50 ppm addition of catalyst in JP-10 reduces the ignition temperature required to initiate combustion by about 240°C | |||
| Catalyst: Hexanethiol monolayer protected Palladium clusters < 1.5 nm | |||
| Application: Hydrogen production by steam reforming of ethanol over nanostructured indium oxide catalysts | |||
| Process Improvements: • At 623K, 99% conversion with mesoporous In2O3/KIT-6 catalyst exhibit high production rates from ethyl alcohol at low-temperatures and yield low concentration of CO impurity in comparison with other reported catalysts | |||
| Catalyst: Mesoporous In2O3 prepared using Mobil Composition of Matter No. 41 (MCM-41) silica catalyst as templates with particle size of 2-3 nm and surface area of 107 m2/g to 173 m2/g | |||
| Application: Adsorptive desulfurization and bio desulfurization of fossil oils | |||
| Process Improvements: In situ coupling desulfurization using assembly of nano adsorbents (nano γ- Al2O3) onto surfaces of Pseudomonas de lafieldii | |||
| Catalyst: Nano γ- Al2O3 (10 nm in width and 100-200 nm in length) with specific surface area of 339 m2/g | |||
| Application: Hydrodesulfurization of diesel | |||
| Process Improvements: Hydrodesulfurization of dibenzothiophene increased by 20% using SDM NiMo/Al-HMS nanocatalyst at 330°C as compared to commercial catalysts. | |||
| Catalyst: Synthesis of new NiMo/Al hexagonal, mesoporous structured nanocomposite catalyst by supercritical deposition method | |||
| Sector: Fuel Cells | |||
| Application: Core-shell nanocatalysts for fuel cell applications | |||
| Process Improvements: • Pt atoms are placed at the surface of other metal nanoparticles • All the Pt atoms are available for catalytic reactions at the surface • Pt clusters on ruthenium nanoparticles produce high activity per unit of Pt mass | |||
| Catalyst: Smooth and compact Pt shell for better oxygen reduction reactions in fuel cell applications | |||
| Application: In situ hydrogen production by reaction of ammonia and nanocatalysts | |||
| Process Improvements: • Ammonia is stored as a coordination complex with a transition metal compound in solid composition • It acts as the hydrogen fuel precursor for a vehicle internal combustion engine that is operated to use hydrogen or a combination of hydrogen and gasoline as fuel • Ammonia dissociation catalyst tube containing a catalyst bed and maintained at 750°C is used to dissociate ammonia into nitrogen and hydrogen atoms | |||
| Catalyst: The dissociation catalyst is a mixture of nanometer size particles of Co-NiO-Cu-Zr catalyst deposited on high surface area of TiO2 and 2% Pt deposited on alumina particles | |||
| Players in the business of nanocatalysis | |||
| Not surprisingly, nanocatalysis is a growing business. The list of companies that have already patented and/or commercialized technologies relating to nanocatalysts is already impressive as exemplified below. | |||
| Headwaters Nanokinetix Inc., NJ, USA | |||
| It has a proprietary patented technology, NxCATTM, that allows catalysts to be designed and engineered at the atomic scale to achieve higher performance and, in some cases, high selectivity with no waste or by products. | |||
| Mach I? Inc., PA, USA | |||
| It has developed the Nanocat? Superfine Iron Oxide (SFIO), an amorphous ferric oxide, which is used as a catalyst for synthesis, cracking and oxidation. It is used in rocket propellants to provide high burning rate, low pressure exponent and safety. Nanocat? SFIO is free of impurities that poison conventional catalysts and is suitable for use in foods, drugs and cosmetics. | |||
| Nanostellar Inc., CA, USA | |||
| It has filed a patent for its Rational Catalyst Design (RCD) technology that uses a unique design approach and computational methods with novel synthesis and testing procedures to manufacture highly efficient nanocatalysts. NS Gold TM, the latest offering from Nanostellar, improves hydrocarbon oxidation by more than 20% over Nanostellar Pt-Pd catalyst and is suitable for heavy-duty trucks, new high efficiency diesel engines that operate at low temperatures, ammonia slip catalysts, VOC and natural gas engines. | |||
| Catalytic Solutions, CA, USA | |||
| It has developed the Mixed Phase Catalyst (MPC?) technology approach that allows manufacturing of catalyst containing stabilized mixed-metal oxides and platinum group metals (PMG). These catalysts resist sintering, or fusing, thereby maintaining a high catalytic surface area and high levels of performance over time using substantially lower PGMs. | |||
| Nanophase Technologies Corp., IL, USA | |||
| Nanophase uses patented coating technology to impart novel functionality to the nanoparticles and for production of nanoparticles. Physical Vapor Synthesis and NanoArc? are two such processes using electric arcs to vaporize precursor materials. | |||
| Quantumsphere?, CA, USA | |||
| It has patented a gas phase condensation process for manufacture of high quality advanced catalyst materials at the nanoscale, including Fe, Ag, Cu, Ni, Co and Mn. | |||
| Hyperion Catalysis International Inc., MA, USA | |||
| The company's FIBRIL Nanotubes are being investigated as an alternative for catalyst supports. These nanotubes are fused together to form a strong highly mesoporous (2-50 nm), open-cell structure that can outperform activated carbon as a highly selective support for precious-metal catalysts. | |||
| NanoScale Corporation, KS, USA | |||
| It has developed a proprietary synthesis method, Nano Active?, for producing metal oxide powders of Al, Ca, Mg, Ti and Zn with the goal of enhancing adsorption kinetics and increasing chemical reactivity in nanocrystalline forms. | |||
| By P. Nagaraju Rao, CKMNT. . | |||
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|||