| Posted: Mar 04, 2011 | |
Multifunctional nanobioprobes detect and isolate multiple types of cancer cells |
|
| (Nanowerk Spotlight) Multifunctional nanomaterials have become widely researched in nanomedicine with the goal of developing highly accurate probes for detecting and isolating cancer cells. Of particular interest here are magnetic nanoparticles, which offer the capability of cell isolation from original or enriched samples without the use of centrifugation or filtration. In particular, the combination of fluorescent quantum dots and magnetic nanoparticles into single nanospheres to obtain fluorescent-magnetic bifunctional nanospheres has created the potential for broader applications in biomedicine and in clinical diagnosis (see for instance "Visual Recognition and Efficient Isolation of Apoptotic Cells with Fluorescent-Magnetic-Biotargeting Multifunctional Nanospheres"). | |
| In new work, researchers in China have now expanded this technique to multiplexed assays. They demonstrated the ability to detect and collect multiple types of cancer cells, such as leukemia cells and prostate cancer cells, from mixed samples within 25 minutes by using a magnet and an ordinary fluorescence microscope. | |
| "Building on years of work, we have now demonstrated that we can successfully detect and extract tumour cells from complex samples containing both normal cells and the target cancer cells," Dai-Wen Pang, Luojia Professor of Chemistry and Director of the Research Center for Nanobiology and Nanomedicine at Wuhan University, tells Nanowerk. "Compared to our previously published work, we used a system of multiple types of rare target cancer cells at concentrations as low as 0.01% in mixed cell samples as a model to demonstrate that our multifunctional nanobioprobe-based analytical method could meet the need for a simple, fast, low-cost, and highly sensitive multi-component assay." | |
 |
|
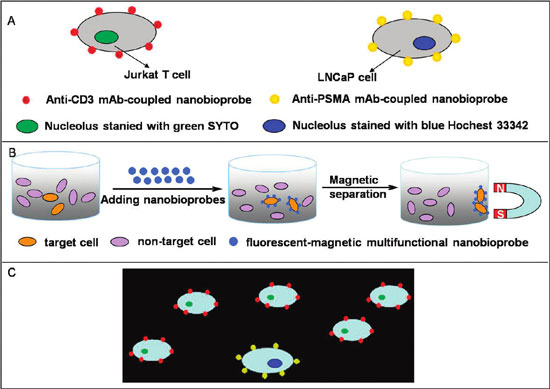
| Schematic drawing of the recognition of specific cancer cells by nanobioprobes. (A) Two types of nanobioprobes coated with anti-CD3 or anti-PSMA mAb recognize Jurkat T cells or LNCaP cells, respectively. (B) Magnetic isolation of cancer cells bound by nanobioprobes. (C) Fluorescent imaging of target cancer cells under a fluorescence microscope. When a mixture of the two types of cancer cells in A was bound by their respective nanobioprobes, they can be distinguished under UV due to different colors of the attached nanobioprobes. (Reprinted with permission from American Chemical Society) | |
| The team reports its findings in the January 20, 2011 online edition of ACS Nano ("Fluorescent-Magnetic-Biotargeting Multifunctional Nanobioprobes for Detecting and Isolating Multiple Types of Tumor Cells"). | |
| While researchers have already succeeded in detecting and isolating cancer cells using fluorescent-magnetic nanoparticles based on receptor-ligand interactions, these studies only used one kind of cancer cells. Detection of multiple tumour cells required sequential process steps with different kinds of nanoparticles. | |
| The novelty of what Pang and his collaborators have demonstrated is the ability to use a single system – monoclonal antibody-coupled fluorescent-magnetic-biotargeting multifunctional nanobioprobes (mAb-coupled FMBMNs) – to identify and isolate multiple target cells from complex mixtures effectively and with high capture efficiency (surpassing 95%). | |
 |
|
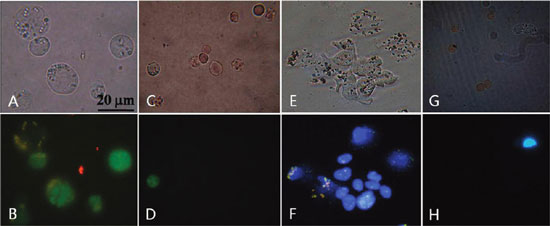
| Fluorescent microscopic images of cells after incubation with mAb-coupled FMBMNs. (A) Bright-field and (B) fluorescent-field images of Jurkat T cells isolated from the mixture of Jurkat T and red blood cells by the anti-CD3 mAb-coupled nanobioprobes in the magnetic precipitate. Note that the Jurkat T cells were labeled green in the nuclei. (C) Bright field and (D) fluorescence field of cells in the supernatant after Jurkat T cells precipitated. Note that few Jurkat T cells (green nuclei) remained. (E) Bright field and (F) fluorescent field of LNCaP cells isolated from the mixture of LNCaP cells and red blood cells by the anti-PSMA mAb-coupled nanobioprobes in the magnetic precipitate. Note that the LNCaP cells were labeled blue in the nuclei. (G) Bright field and (H) fluorescent field of cells in the supernatant after LNCaP cells precipitated. Note that few LNCaP cells (blue nuclei) remained. (Reprinted with permission from American Chemical Society) | |
| "Under our experimental conditions, the nanobioprobes could specifically detect and isolate target cells at concentrations as low as 0.01% in mixed cell samples," says Pang. "This suggests the possibility of such nanobioprobes for clinical applications in the early detection of cancer cells." | |
| He points out that these fluorescent-magnetic-biotargeting multifunctional nanobioprobes can not only be used for detecting and isolating multiple types of tumour cells, but also have a promising potential in the fields of fluorescent-and-magnetic multimodal imaging for early diagnosis of diseases, imaging-coupled drug delivery for treatment of cancer diseases, and monitoring therapeutic responses in real time. | |
| "However, before their clinical application, the issues of biosafety, in vivo targeting efficacy, and long-term stability of the multifunctional nanobioprobes need to be intensively and systematically investigated," says Pang. Something the team is already working on. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
