| Posted: Jun 08, 2007 | |
Ethanol production inside carbon nanotubes |
|
| (Nanowerk Spotlight) Ethanol is all the rage these days. Although we have been drinking ethanol, an alcohol, for thousands of years (fermented beverages such as beer and wine may contain up to 15–25% ethanol by volume), the recent interest has been sparked by its use as a renewable fuel alternative to gasoline. Indeed, the largest single use of ethanol is as a motor fuel and fuel additive. | |
| Ethanol is produced by fermentation when certain species of yeast metabolize sugar. The process works with all biological feedstocks that contain appreciable amounts of sugar or materials that can be converted into sugar such as starch or cellulose. The primary feedstock for ethanol production in the U.S. is corn. In Brazil, the world's leading ethanol producer, it's mostly derived from sugar cane. | |
| While there is a heated controversy over the economic and ecological benefits of using biomass for producing ethanol fuel, it seems that nanotechnology's jack-of-all-trades, the carbon nanotube (CNT), might provide a solution here as well. CNTs are increasingly recognized as promising materials for catalysis, either as catalysts themselves, as catalyst additives or as catalyst supports. Researchers in China now have used CNTs loaded with rhodium (Rh) nanoparticles as reactors to convert a gas mixture of carbon monoxide and hydrogen into ethanol. This appears to be the first example where the activity and selectivity of a metal-catalyzed gas-phase reaction benefits significantly from proceeding inside a nanosized CNT reaction vessel. | |
| CNTs distinguish themselves from other carbon materials, e.g. activated carbon and carbon nanofibers, in that they have well graphitized graphene with semiconducting or metallic characteristics and a tubular morphology with well defined dimensions. Earlier theoretical studies have shown that the electron density is shifted from the inside to the outside of CNT channels, and that inside gas molecules exhibit a binding energy different from those outside of the nanotubes. | |
| "We were curious about what would happen if we combined these graphene tubes with metal nanoparticles, which have interesting redox and catalytic properties by themselves" Dr. Xinhe Bao tells Nanowerk. "We previously found that the redox properties of iron and iron oxide particles are tunable via encapsulation within CNTs." | |
| Bao, a professor at the State Key Laboratory of Catalysis at the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and head of the institute's Nano and Interfacial Catalysis Group, found that, for instance, iron oxide particles within 4-8 nm wide nanotubes are auto-reduced at 600 degrees Celsius while the particles located on the outer surface of the nanotubes need 800 degrees Celsius. Furthermore, the auto-reduction temperature of inside particles decreases with the nanotube diameter. On the other hand, the oxidation of metallic iron nanoparticles is retarded inside nanotubes compared to those particles located on the outer surface of nanotubes. Both experiments indicate the modification of the redox properties of these particles inside CNTs and the stabilization of metallic Fe inside nanotubes ("Tuning of Redox Properties of Iron and Iron Oxides via Encapsulation within Carbon Nanotubes"). | |
| "We suspected that the modification of the redox properties of metal particles inside CNTs is a general characteristic and that this could be exploited in catalysis" adds Dr. Xiulian Pan, a member of Bao's group and first author of the team's recent publication in Nature Materials, where they describe their CNT ethanol production method ("Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles"). | |
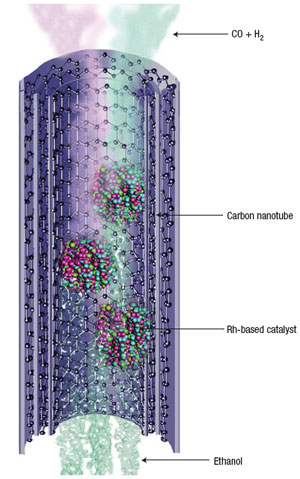 |
Schematic diagram showing ethanol production from syngas inside Rh-loaded carbon nanotubes. The black spheres denote carbon atoms, which form the graphene layers of the carbon nanotubes. The streams in light orange and green entering the nanotubes indicate the gas mixture of CO and H2, respectively. The three stacks of small spheres in rose, blue, green and red inside the tubes represent catalyst particles that may comprise more than one component. The streams in light cyan tailing behind the catalyst particles along the axis of the nanotubes represent ethanol. (Reprinted with permission from Nature Publishing Group) |
| "Therefore, we introduced a promoted RhMn catalyst for syngas conversion into carbon nanotube channels" she says. "Syngas is a 1:2 mixture of CO and H2. This reaction is known to be very sensitive to the redox states of Rh and Mn. Oxygenates containing two carbon atoms such as ethanol, acetyldehyde and acetic acid were produced, and surprisingly, the yield over the CNT-encapsulated catalyst was extraordinarily high, clearly exceeding that of the very good silica-supported catalyst. Furthermore, catalysts with metal particles confined inside CNTs were also significantly more active than those with the metal particles dispersed on the outer surface of the nanotubes, even though the latter are more easily accessible." | |
| The results of the Chinese scientists suggest a host-guest interaction between the confined metal particles and CNTs, which is different from that on the outside of the nanotubes. Other effects may also play a role, like the stringent size restriction of metal particles inside CNTs and the high affinity of hydrogen to the inner surfaces of opened CNTs as exemplified in their extraordinary hydrogen adsorption capacity. Pan says that she believes that other conversions could benefit in a similar way from taking place inside CNTs, in particular if they involve hydrogen. "We also anticipate that the study of the host-guest interaction within CNTs will attract greater attention as a result." | |
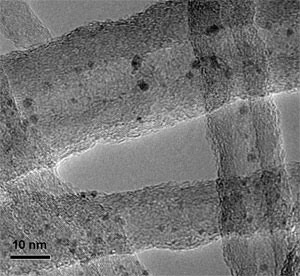 |
TEM image of the Rh-based catalyst particles encapsulated within the carbon nanotube channels. (Image: Dr. Bao, Dr. Pan) |
| Bao points out that experimental study of the redox properties and the electronic host-guest interaction in these systems is still a challenge and might require refined characterization techniques. "Other effects may also play a role in these catalysts" he says, "like the stronger size restriction of metal particles inside CNTs and the high affinity of hydrogen to the inner surfaces of opened CNTs. The understanding and distinction between these contributions needs to be advanced by further experimental and theoretical studies. Besides, we are currently looking at new experimental characterization techniques which provide deeper insight into the nature of these confined systems." | |
| Pan notes that, apart from the still considerable challenge of cost efficient, large-scale production of CNTs with precise diameter and chirality control, a further challenge pertaining to catalysis is the homogeneous dispersion of metal nanoparticles within the CNT channels, since this can strongly influences the activity of these catalysts. | |
| Apart from applications in catalysis, such CNT encapsulates might also be interesting for composite materials which require a modulation of the electronic state, such as magnetic sensor or storage materials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
