| Posted: May 17, 2011 | |
The ideal microfluidic point-of-care device |
|
| (Nanowerk Spotlight) Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. To analyze the presence and level of certain biomarkers in body fluids, miniaturized immunoassays that make use of microfluidics have become an important analysis technique. | |
| Microfluidic chips have proven to be a breakthrough analytical technique that has rendered analysis of proteins a medical routine. The sensitivity limits of immunoassays have been enhanced to picomolar concentrations using monoclonal antibodies, new labeling techniques, and devices for signal transduction and acquisition. It is now possible to routinely determine levels of hormones, cancer markers, response to infection with bacteria and viruses, monitor the evolution of a disease and test for medication levels. | |
| In a new review article in Advanced Materials ("Microfluidic Chips for Point-of-Care Immuno-diagnostics"), Luc Gervais and Emmanuel Delamarche from IBM Zürich Research Laboratory and Nico de Rooij from the Institute of Microengineering at École Polytechnique Fédérale de Lausanne argue that the most promising opportunities of microfluidics for diagnostics reside in point-of-care (POC) applications – i.e. in medical testing at or near the site of patient care – because a number of unmet needs can be fulfilled by microfluidic devices due to their portability, short sample processing time, and flexibility. | |
| In their paper, the Swiss researchers brainstorm on what the ideal microfluidic POC device would be: | |
| "Arguably, the ideal POC diagnostic device would use a small volume of unprocessed sample taken directly from the patient. This volume could be as low at 1 µL, which is significantly less than a drop from a finger prick (typically 25 µ L) and is minimally invasive, especially when used with rare samples and patients such as premature babies, newborns, adults suffering from anemia, and the elderly. Accurate sampling would be done by the chip and tests would have the possibility to use an increased volume. The device would multiplex the analysis of up to a 100 analytes that would be a variety of proteins and nucleic acids. Quantitative results would be obtained within 1 minute. The sensitivity limit would be in the picomolar to femtomolar range for detection of analytes present in low concentrations. The dynamic range of the device would be large, detecting up to micromolar quantities of analytes that have a large concentration range. Analytes would be detected with great selectivity, eliminating cross talk and false positives. Negative controls would also be included. There would be no cross contamination between samples from different patients or different runs." | |
| They continue their wish list: "The rugged device would be impermeable to water and not damaged when dropped from one meter above ground. It would be made of transparent material where flow should be monitored and optical signal stimulated and read. The device would have a long battery life and could be used for years before recharging. The device would have a shelf life of years when stored between -55°C and 55°C without a reduction in performance. The device would be safe to use in a hospital setting, user-friendly and easy to use by non-technical experts. The device would analyze the sample, calibrate the result, record and transmit encrypted data wirelessly to an electronic health record. To satisfy all actors in the world of diagnostics, except maybe the manufacturer, the disposable part of the device would cost less than one dollar to fabricate. In other words, to be competitive the test would be in the same cost range as the ubiquitous immunoassay strip test." | |
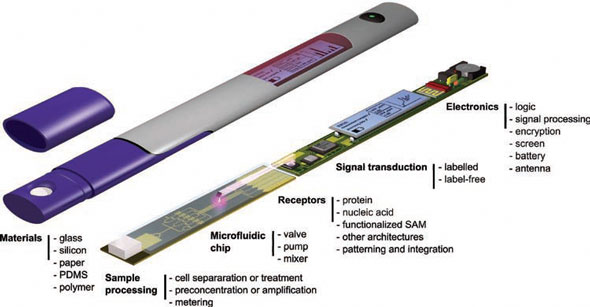 |
|
| The ideal POC diagnostic device. The ideal POC device quantitatively detects several analytes, within minutes, at femtomolar sensitivity from 1 µL of bodily fluid and reports the encrypted results to an electronic health record. The device would have the possibility to use an increased volume of sample with very low concentrations of analytes. The microfluidic chip, shown here encapsulated in purple plastic, is disposable and the mass manufacturing material cost would be less than $1. This ideal POC device does not exist but research progress in microfluidics and material science point toward the realization of such a POC device in the near future. (Source: Advanced Materials, © 2010 IBM Corporation) | |
| The ideal POC device imagined by the Swiss team does not exist but they say that by crystallizing a vision around it helps identifying desirable characteristics of this device. They then go on and put these characteristics in the context of recent progress in the fields of microfluidics and material science. | |
| "Precise control of materials is essential to realize such a properly working POC device" they write. "An antifouling surface, wettability, filtration/processing of the sample, flow control, advanced signal generation principles, receptor attachment and assembly are all highly dependent on the properties of materials." | |
| In reviewing the state of microfluidic chips and immunoassays, the authors focus on discussing recent progress in microfluidic components, biology, materials and hardware. They do not yet describe the information technology part of the device but they review the functional elements that are relevant for POC diagnostic devices such as the ideal one discussed in their article. | |
| One obvious question that the authors ask is why POC diagnostics isn't a killer application for microfluidics if we already have most of the technology and know-how at our disposal to make these devices? The seem to be several reasons for this: | |
| "The development and adoption of microfluidic-based POC diagnostics is impacted by the segmented nature of the diagnostics market, major existing clinical technologies, fierce competition in the market, strong regulation from public health agencies, and patents on reagents (such as antibodies and biomarkers) and technology components. The reimbursement of diagnostic devices, their acceptance by medical personnel (users), business models that are rarely intuitive, large geographical disparities and medical practices add to the complexity of deploying new technologies." | |
| A solution to all these challenges is probably to identify key analytical needs in the life sciences and to create reference applications involving microfluidic technologies. | |
| The authors conclude that, in their opinion, microfluidic diagnostics devices are on the brink of supporting decentralized testing. | |
| "These devices will enable patients to monitor themselves outside of the hospital and communicate results with clinicians, ultimately giving patients a greater control of their own health and access to their personal health data. In addition, these devices may also support the emergence of personalized medicine in which treatments are tailored to the genetic profile of patients and/or to their specific metabolism of drugs." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
