| Posted: May 31, 2011 | |
A coating for jet engines to prevent volcanic ash damage |
|
| (Nanowerk Spotlight) First there was Eyjafjallajökull, now there is Grímsvötn. Back in April 2010, the ash clouds from the Icelandic volcano Eyjafjallajökull caused the longest no-fly period in European air space. Hundreds of thousands of travelers were stranded, airlines suffered huge losses, and supply lines were seriously squeezed. Last week, there was some kind of déjà vu when Grímsvötn, historically the most active Icelandic volcano, erupted and countries started to close their airspace again. | |
| The problem is that the small particles (less than 2 mm) in volcanic ash can damage jet aircraft engines. During jet engine operation, large amounts of air are sucked in. If this air contains ash particles, especially glass-rich silicate ash, they will melt in the jet turbine's operating temperature of 1400-1500°C and subsequently fuse to the turbine blades. This will drive the finely tuned blades out-of-balance and could stall the engine. | |
| In today's jet engines, a thermal barrier coating (TBC) insulates metallic engine parts from the heat generated by the combustion process. If ingested ash melts onto this coating it will penetrate it and, upon cooling, the molten ash forms a brittle glass that flakes off, taking the coating with it. | |
| A team of researchers have now examined a new class of ceramic TBC that could offer jet engines special protection against volcanic ash damage in the future. Reporting their findings in the June 3, 2011 issue of Advanced Materials ("Jet Engine Coatings for Resisting Volcanic Ash Damage") | |
| Doctoral students Julie Drexler and Andrew Gledhill from Nitin P. Padture's research group in the Department of Materials Science and Engineering at Ohio State University took samples of the ceramic coatings on pieces of metal, and coated them with ash from the Eyjafjallajökull eruption. Then they heated the samples in a furnace to simulate the high temperatures created in a jet engine. They experimented with a typical jet engine coating – TBC is typically made of tetragonal-phase ZrO2 ceramic stabilized by 7 wt% (3.9 mol%) Y2O3 in solid solution (7YSZ). – and a sand-resistant coatings previously developed by Padture, containing zirconia and alumina. | |
| In that test, the ash badly damaged the typical coating, while coatings made of Padture's formula (as well as another sand-resistant coating made with a gadolinium zirconate formula) retained their overall structure. Looking at cross-sections of the samples, the researchers saw why: molten ash had penetrated through the pores of the typical ceramic coating all the way to its base. But in the other two, the molten ash barely penetrated. | |
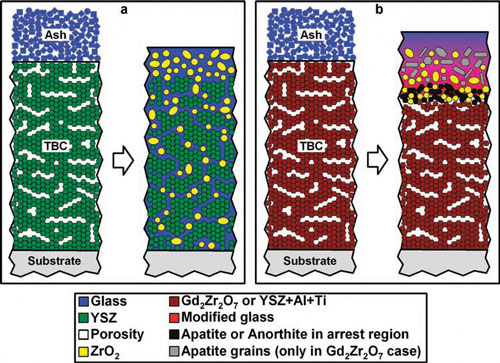 |
|
| Schematic diagrams of APS TBCs cross-sections with ash deposits, before and after exposure to heat. (a) 7YSZ and (b) Gd2Zr2O7 or YSZ+Al+Ti. APS TBCs are polycrystalline, with pores and cracks generally running parallel to the TBC/substrate interface. (a) In 7YSZ TBC the ash melts to form a glass that penetrates pores, cracks and grain boundaries, while 7YSZ grains dissolve and reprecipitate as rounded ZrO2 grains (yellow) depleted in Y-content. (b) In Gd2Zr2O7 or YSZ+Al+Ti TBCs the formation of an impervious, crystalline reaction layer consisting of rounded ZrO2 grains together with apatite-type phase or anorthite prevents further penetration of molten ash. Isolated faceted apatite-type grains in a glassy matrix above the reaction zone in (b) are observed only in the Gd2Zr2O7 TBC case. Diagrams not to scale. (Reprinted with permission from Wiley-VCH Verlag) | |
| "Pores give the coating its strain tolerance," explains Drexler. "They make room for the coating to expand and contract as the engine heats up while flying, and as it cools after landing. When all the pores are plugged with ash, the coating can't adjust to the temperature anymore, and it breaks off." | |
| On the sand-resistant coatings, the ash filled the pores only near the surface. Chemical analysis revealed that the ash reacted with the alumina in the first coating to produce a thin layer of the mineral anorthite below the surface, while on the gadolinium zirconate it produced a layer of the mineral apatite. | |
| "The chemical reaction arrests the penetration of the ash into the coatings," says Gledhill. "The unaffected pores allow the coating to expand and contract." | |
| While this study is limited to a specific sample of Eyjafjallajökull volcanic ash, the researchers note that it serves as a proof-of-concept and provides motivation for tailoring TBC compositions to mitigate attack by molten ashes of other relevant compositions. They caution, though, that the long-term thermo-mechanical performance of these TBCs experiencing thermal gradients and complex temperature excursions in the presence of volcanic ash is yet to be determined. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
