| Posted: Nov 02, 2011 | |
Novel graphene electrode improves already promising lithium-air battery technology |
|
| (Nanowerk Spotlight) Lithium-ion batteries have been widely used in many electronic devices that are important to our daily life. However, after a steady improvement of some 10-15% during the last two decades, the energy density of lithium-ion batteries is now approaching its theoretical limit set by the energies of cathode and anode materials used in these batteries. Therefore, in recent years, the pursuit of the next generation of energy storage systems has been intense globally. | |
| One such system is metal/air batteries, which have much higher specific energies than most currently available primary and rechargeable batteries. | |
| "Metal/air batteries are unique in that the cathode active material is not stored in the battery" Dr. Ji-Guang Zhang, a researcher at Pacific Northwest National Laboratory's Transformational Materials Science Initiative, explains to Nanowerk. "Instead, oxygen from the environment is reduced by catalytic surfaces inside the air electrode, forming either an oxide or peroxide ion that further reacts with cationic species in the electrolyte. The Li/O2 couple is especially attractive because it has the potential for the highest specific energy among all the known electrochemical couples." | |
| Among various electrochemical energy storage systems explored to date, the lithium-air (Li-air) battery is one of the most promising technologies, with a theoretical energy density nearly ten times that of conventional lithium-ion batteries. This is because lithium metal as an anode has a capacity ten times higher than that of conventional graphite anodes, and oxygen as the cathode of a Li-air battery can be absorbed freely from the environment leading to a significant reduction in the weight and the cost of the battery. | |
| For use in practical devices such as electric cars, researchers expect that Li-air batteries achieve an energy density of about 800 Wh/kg – which is three times as large as those of the state of the art Li-ion batteries. Therefore, Li-air batteries have a good potential to be used in many applications which requires an energy storage system beyond those of Li-ion batteries, such as long range electrical vehicles which can run more than 500 kilometers per charge. | |
| Reporting their results in the October 10, 2011 online edition of Nano Letters ("Hierarchically Porous Graphene as a Lithium-Air Battery Electrode"), Zhang and his PNNL team demonstrate that a novel air electrode consisting of an unusual hierarchical arrangement of functionalized graphene sheets (with no catalyst) delivers an exceptionally high capacity of 15000 mAh/g in lithium-O2 batteries – which is the highest value ever reported in this field. | |
| The performance of Li-air batteries is affected by many factors such as electrolyte composition, the macrostructure of the air electrode, and the micro- to nanostructure of carbonaceous materials. Precipitation of reaction products (such as Li2O2) on the carbonaceous electrode eventually blocks the oxygen pathway and limits the capacity of the Li-air batteries. | |
| This recent work by the PNNL team minimizes air-electrode-blocking problem and leads to significantly increased capacities. | |
 |
|
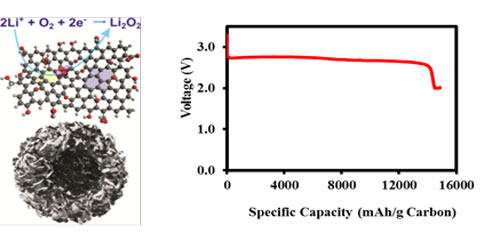
| (a) Schematic structure of functionalized graphene sheet (upper image) with an ideal bimodal porous structure (lower image) which is highly desirable for Li-O2 battery operation. (b) The discharge curve of a Li-O2 cell using FGS(C/O = 14) as the air electrode (PO2 = 2 atm). (Figure: Dr. Ji-Guang Zhang, Pacific Northwest National Laboratory) | |
| "Most of previous works used mesoporous carbons or graphene sheets cast into a stacked two-dimensional structure which limited its accessible capacities," Zhang says. "Our work is first to demonstrate a self-assembled, bimodal graphene structure with both micron-sized open porosity for rapid oxygen diffusion and substantial nanoporosity (2-50 nm) to catalyze Li-O2 reactions while preventing excessive growth of the discharge products that block chemical pathways." | |
| Further, the researchers show that the defects and functional groups on graphene favor the formation of isolated nano-sized Li2O2 particles and help prevent air blocking in air electrode. The hierarchically ordered porous structure in bulk graphene enables its practical applications by promoting accessibility to most graphene sheets in this structure. | |
| To produce their air electrodes based on functionalized graphene sheets, the functionalized graphene sheets were first dispersed in a microemulsion solution which also contained the binder materials for the electrode. After casting and drying, very unusual morphologies were produced. | |
| "Surprisingly, the functionalized graphene sheets aggregated into loosely packed, 'broken egg' structures leaving large interconnected tunnels that continue through the entire electrode depth," says Zhang. "These tunnels can function as numerous arteries that continuously supply oxygen into the interior of the electrode during the discharge process. More importantly, the complex pore structure is retained after electrolyte infiltration, unlike other porous carbon materials tested." | |
| When they investigated the 'shells' of the 'broken eggs' by SEM, the researchers found that the 'shells' consist of numerous smaller nanoscale pores contiguous with the large tunnels. As Zhang points out, this unique morphology is an ideal design for an air electrode. "During discharge the robust large tunnels can function as 'highways' to supply the oxygen to the interior parts of air electrode while the small pores on the walls are the 'exits' which provide triphase (solid-liquid-gas) regions required for oxygen reduction." | |
| Several barriers still need to be overcome before this battery system could be applied at a large scale. The main barriers include: Low discharge rate; electrolyte stability and Li-air cell reversibility; oxygen selective membrane is needed to reduce the capacity fade due to moisture penetration; and lithium dendrite growth need to be prevented to extend the cycle life. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
