| Posted: Aug 13, 2007 | |
Nanoscopy - nanoscale resolution in light microscopy |
|
| (Nanowerk Spotlight) In the early 1870s, the German physicist Ernst Karl Abbé formulated a rigorous criterion for being able to resolve two objects in a light microscope. According to his equation, the best resolution achievable with visible light is about 200 nanometers. This theoretical resolution limit of conventional optical imaging methodology was the primary factor motivating the development of recent higher-resolution scanning probe techniques. | |
| The interaction of light with an object results in the generation of what is called 'near-field' and 'far-field' light components. The far-field light propagates through space in an unconfined manner and is the visible light utilized in conventional light microscopy. The near-field (or evanescent) light consists of a nonpropagating field that exists near the surface of an object at distances less than a single wavelength of light. | |
| So called near-field microscopy beats light's diffraction limit by moving the source very close to the subject to be imaged. When the first theoretical work on a new technique called "scanning near-field optical microscopy" (SNOM or NSOM) appeared in the 1980's, Abbé's classical diffraction limit was overcome, and resolution even down to single molecule level became feasible. However, light microscopy is still the only way to observe the interior of whole, or even living, cells. | |
| The use of fluorescent dyes makes it possible to selectively obtain images of individual cell components, for example, proteins. Today, the wavelength dogma has been overcome with the development of the stimulated emission depletion (STED) microscope. Now, the German team that developed STED is reporting layer-by-layer light microscopic nanoscale images of cells and without having to prepare thin sections with a technique called optical 3D far-field microscopy. They use a chemical marker for fluorescence nanoscopy that relies on single-molecule photoswitching. | |
| "Well thought-out modifications of the fluorescence process have opened a back-door for circumventing Abbé's paradigm" explains Dr. Stefan Hell, head of the nanobiophotonics department at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany. "In a scanning microscope, improving the resolution is equivalent to decreasing the spatial extent of the fluorescent spot that is scanned through the sample. Hence, we are scrutinizing physical phenomena that bear the potential of cutting down the size of the fluorescence spot. The focal spot is termed 'the point-spread-function'. So we dubbed this down-sizing approach of the spot as Point-Spread-Function Engineering ("Increasing the Resolution of Far-Field Fluorescence Light Microscopy by Point-Spread-Function Engineering"). But non-scanning microscopes have Point-Spread-Functions, too. In fact virtually all of the physical ingredients that we use in a scanning microscope can be employed in a non-scanning CCD camera based system as well, with some modifications and advantages and disadvantages." | |
| Hell explains that the states of the fluorescent marker was not just used for generating the signal, but also for breaking the diffraction barrier. "In fact, all the methods that have successfully outperformed diffraction have so far relied on selected pairs of molecular states – specifically, a 'bright' one to generate the signal and a 'dark' one to ensure that the measured signal stems from a subdiffraction-sized region." | |
| "An alternative way of using molecular photoswitching to break the diffraction barrier is to stochastically switch on, read out the fluorescence, and switch off isolated marker molecules such that simultaneously emitting ('on') markers are further apart than the minimal distance resolved by the microscope" says Hell. "In this case, the spatial confinement of the fluorescence is down to the size of a single molecule by definition." | |
| Hell's group has now been demonstrating the power of this concept. They report the use of molecules that are not only transferred but can be 'switched' from fluorescent to non-fluorescent and back ("Photochromic Rhodamines Provide Nanoscopy with Optical Sectioning"). | |
 |
|
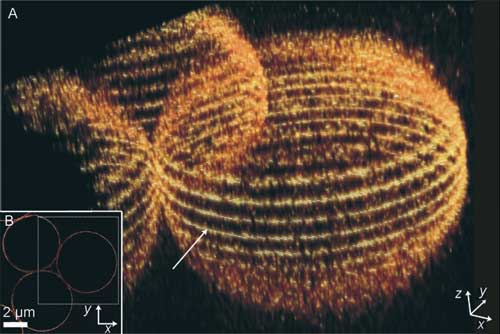
| A, B) Imaging of 5-mm silica beads surface stained with 5-NHSS. A) 3D reconstruction from 17 slices in the z direction; B) equatorial slice (arrow in (A)). For clarity, a smaller area was selected for the 3D reconstruction (dotted-line box in (B)). (Reprinted with permission from Wiley-VCH Verlag) | |
| In contrast to STED, only separate, isolated marker molecules are randomly switched on at the same time. Their fluorescence is registered, and then they get switched off again automatically. In this way, the simultaneously fluorescing (switched on) markers are farther apart from each other than the minimum distance that the microscope can resolve. | |
| This is only possible using switchable molecules that emit many photons, one after the other, when switched on. If these photons are captured with a camera, the centers of the individual fluorescing dots can be distinguished. After the exposure, the molecule becomes dark again (switches off), allowing further, neighboring molecules to be photographed. This process is repeated many times, until many dots become a picture. The full distribution can be reconstructed – at a resolution not limited by the wavelength of light. | |
| The researchers have now found a class of substances that fulfill all the requirements of this technique: rhodamine amides. At the core of these molecules lies a system of five rings. In this form, the compound is colorless and does not fluoresce. Irradiation with light induces an isomerization in which one of the rings is opened. This form of the molecule is red and can be excited several times. | |
| Most importantly: rhodamine amides can be switched on by either a UV photon or two photons in the red part of the spectrum. This two-photon excitation can be focused onto a thin plane, which allows biological samples to be photographed layer by layer. The individual images can then be reconstructed into a single multilayer image. The resolution reached in the focal plane is far beyond the diffraction barrier (10–30 nm). | |
| Hell has also published a comprehensive review of the state of far-field optical nanoscopy in a recent article in Science. In it he concludes: "Along with improved fluorescence schemes, nonfluorescent schemes should enable us to take another step in the not-too-distant future: nanoscale 3D imaging at high speed. In any case, the works reviewed herein already broke the barrier of perception of what a lens-based light microscope is able to accomplish. With human perseverance focused on this matter, 3D imaging of live cells with electron microscopy resolution should be possible." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
