| Posted: Dec 01, 2011 | |
Nanotechnology could make battery recycling economically attractive |
|
| (Nanowerk Spotlight) Batteries are an integral part of modern life – just go ahead and count the batteries that you use yourself in your watches, computers, cell phones, cameras, alarm clocks, flashlights, toys, remote controls, power tools, cars, boats and so on. You'll come up with a staggering number. And chances are that your batteries are disposable, so you throw them out with your garbage when they are empty. Add to that the batteries used by industry, hospitals, public transport, the military etc and you get several billion batteries that are bought every year, a roughly $50 billion market. | |
| Many batteries still contain heavy metals such as mercury, lead, cadmium, and nickel, which can contaminate the environment and pose a potential threat to human health when batteries are improperly disposed of. Not only do the billions upon billions of batteries in landfills pose an environmental problem, they also are a complete waste of a potential and cheap raw material. | |
| Unfortunately, current recycling methods for many battery types, especially the small consumer type ones, don't make sense from an economical point of view since the recycling costs exceed the recoverable metals value. Therefore, recycling companies only take up spent batteries if someone pays for their service. In Switzerland, for instance, the purchase price of batteries up to a weight of 5 kg contains the cost for the battery's disposal which finances the entire recycling process. | |
| The economic recycling problem is particularly serious in developing countries like India where, so far, economic interests supersede environmental obligations. This situation makes the development of economically interesting battery recycling technologies quite an urgent issue. | |
| "Most of the reported processes for the recovery of metals from spent batteries focuses on production of metal salts/oxides/ferrites," Akash Deep, a scientist in the Biomolecular Electronics and Nanotechnology Division at the Central Scientific Instruments Organization in Chandigarh, India, explains to Nanowerk. "Steps involved in these technologies include ammoniacal or acidic leaching, precipitation, solvent extraction, and thermal treatment. Generally the recycling of lead-acid, lithium ion and rechargeable Ni-Cd batteries is given more importance. But, in reality, the relative consumption of alkaline Zn-MnO2 batteries is much larger." | |
| Deep and his team carried out research to address the recycling of consumer-type batteries. Reporting their findings in a recent issue of Environmental Science & Technology ("Recovery of Pure ZnO Nanoparticles from Spent Zn-MnO2 Alkaline Batteries"), they describe the recovery of pure zinc oxide nanoparticles from spent Zn-Mn dry alkaline batteries. | |
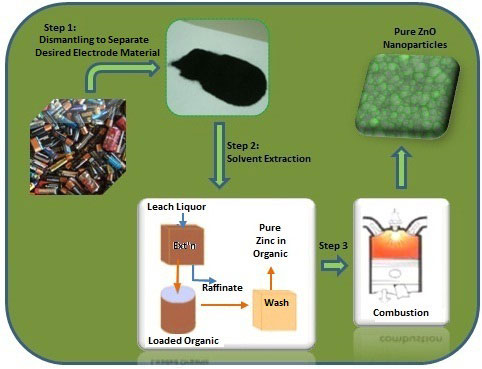 |
|
| Scheme for the recovery of pure zinc oxide nanoparticles from spent Zn-Mn dry alkaline batteries. (Image: Dr. Deep, Central Scientific Instruments Organization) | |
| The researchers dismantled the spent batteries and leached the desired metals from the waste electrode materials. They introduced a solvent extraction step (using the acid extraxtant Cyanex 923) to selectively extract zinc from the powderized electrode materials. | |
| "This commercially available extractant is cheaper than the previously tried Cyanex 272/Cyanex 301, offers clearer phase separations and better loading capacity, and is easy to regenerate," says Deep. | |
| After extracting the zinc, the team incinerated the pure zinc loaded organic layer at 600°C for the synthesis of high-purity zinc oxide nanoparticles. | |
| "The recovered pure zinc oxide nanoparticles may find applications in piezoelectric transducers, gas sensors, photonic crystals, light-emitting devices, photodetectors, photodiodes, optical waveguides, transparent conductive films, varistors, and solar cells," says Deep. | |
| Based on the team's calculations, the cost estimation for the production of 1.23 kg of high purity zinc oxide (1 kg equivalent zinc) nanoparticles using their recycling process shows a positive result: | |
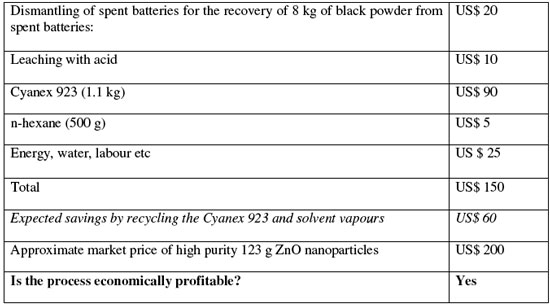 |
|
| Although this present work focuses on the recovery of pure nanoparticles from spent alkaline Zn-MnO2 batteries, the same approach may be utilized in recycling of other types of spent batteries, including Ni-Cd, Li-ion, Zn-C, and Pb-acid. | |
| "Our research team is working on the recovery of pure cadmium and lead nanoparticles and trying to develop green processes for the ultimate recovery of highly pure and efficient zinc, cadmium, and lead quantum dots from environmental waste," Deep describes the group's current research focus. "In future, we are expecting to turn the different environmental wastes, such as spent batteries, electronic parts, LCD and LED panels, or semiconductor waste into lucrative raw materials for the recovery of high cost nanoparticles, quantum dots, nanowires etc." | |
| Over the past two decades, the advancements in nanotechnologies have spurred the use of metallic and non-metallic nanoparticles in virtually every scientific field. There is an increasing demand of customized nanoparticles, nanowires, nanotubes, atomic layers and other similar kind of photonically or electrically active nanotemplates. | |
| Combine this with the environmental implications of the various nanofabrication routes (see: "Nanotechnology – not that green?") and you'll see a trend where nanotechnologies actually build up quite a significant ecological footprint. | |
| However, research like the one done by Deep and his collaborators will appeal to environmental nanotechnologists who should see this as an opportunity to turn recycling of electronic waste into an economically beneficial proposition for industry. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
