| Posted: Aug 14, 2007 | |
Microbotics - nanoparticles hitching a ride on bacteria |
|
| (Nanowerk Spotlight) Vaccination has resulted in the eradication of smallpox and control of measles, rubella, tetanus, diphtheria, and other infectious diseases in many areas of the world (at least where vaccines are available and affordable; providing vaccines to many parts of the developing world still is one of the basic medical needs that is far from being met). The basic idea of vaccination (the word comes from the Latin vacca - cow - because the first vaccine was derived from a virus affecting cows) is to inject weakened or killed forms of pathogens such as bacteria or viruses into the body in order for the immune system to develop antibodies against them; if the same types of microorganisms enter the body again, they will be destroyed by the antibodies. About 25 years ago, the basic idea of vaccination gave rise to bactofection - the technique of using bacteria as non-viral gene carriers into target cells. The DNA cargo is transported inside the bacteria and, once it arrives at the target location, the bacteria is broken up in order to release the therapeutic gene or protein. A novel technique takes advantage of the invasive properties of bacteria for delivery of nanoparticles into cells. Here, the gene or cargo is not carried inside the bacteria, but rather remains on the surface conjugated to nanoparticles. Consequently, this approach does not require bacterial disruption for delivery, or any genetic engineering of the bacteria for different cargo. | |
| "Our bacteria-mediated nanoparticle and cargo delivery approach, which we term microbotics, promises excellent potential for nonviral gene delivery, and unique capabilities for biomedical nanorobotics and nanomedical therapy" explains Dr. Rashid Bashir. "Although more than one gene can be delivered by means of bactofection, many more copies of a target cargo can be carried with one bacterium using our method. We also show that nucleic acid-based model drugs loaded on the nanoparticles can be released from the carriers and eventually find their way into the nucleus, with subsequent transcription and translation of their respective proteins, for both in vitro and in vivo conditions. Such bacteria, which we call microbots, can potentially be used to carry proteins, small molecules and even synthetic objects like sensors and therapeutic moieties into different types of cells." | |
| Bashir, Professor of Electrical and Computer Engineering at Purdue's Birck Nanotechnology Center, together with colleagues from various departments at Purdue University, published a paper in Nature Nanotechnology where they describe a clever use of microbiology and nanotechnology to use bacteria to deliver nanoparticles into cells ("Bacteria-mediated delivery of nanoparticles and cargo into cells"). | |
| Bashir's team coupled polystyrene nanoparticles loaded with plasmid DNA to the surface of weakened Listeria monocytogenes bacteria. | |
 |
"Three steps were necessary to make our microbots" says Bashir: "First we treated the bacteria with a biotin-carrying antibody that acts against — and will therefore attach to — proteins on the bacterial surface called muraminidase. Next, we mixed the treated bacteria with nanoparticles, ranging from 40nm to 200 nm, coated with streptavidin, a protein that binds strongly to biotin. Finally, the nanoparticle-loaded bacteria were mixed with plasmid DNA carrying biotin, which binds to the free streptavidin sites on the surface of the nanoparticles." |
Because the nanoparticles are linked to the bacteria by means of an antigen–antibody interaction, the cargo and the bacteria can readily separate in the lower pH environment of the subcellular compartments. Rashid says that other factors, such as intracellular enzymatic processing or destabilization of antigen–antibody binding or a reduction in the biotin– streptavidin interactions can also be involved in the release mechanisms of the DNA, and all of these possibilities can potentially be used for endowing microbots with smart cargo release ability. |
|
In their experiments, the researchers at Purdue found that microbots successfully delivered their cargos of nucleic acid-based model drugs, plasmid DNAs for firefly luciferase and SEAP enzymes into multiple organs of live mice, and the delivered genes also resulted in functional protein expression by three days post-treatment. |
|
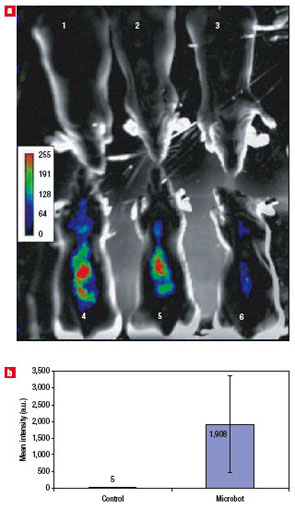
| Microbot-mediated delivery and functional expression of luciferase gene in mice. a) In mice whole-animal bioluminescence images of mice with microbots carrying the firefly luciferase gene at three days post microbot treatment. Note the significant increase in photons collected from the microbot-treated animals (4?6) compared with the PBS-treated (sham-control) animals (1?3). The mice are in the ventro-dorsal position. b) Quantification of bioluminescence in sham-treated (white bar) and microbot-treated (blue bar) mice from a. On average, an 380-fold increase in bioluminescence was observed in microbot-treated animals compared with PBS-treated mice (n=3 animals per group, P<0.01). Error bars represent standard deviations.. (Reprinted with permission from Nature Publishing Group) | "The delivered plasmid DNAs were able to escape from intracellular entrapment and were targeted to the nuclei of the cells, resulting in transcription and expression of the enzymes" says Bashir. "Hence, our novel technology can be used to deliver these reporter molecules for whole-animal live imaging agents (luciferase) or for non-invasive in vivo reporter assays (SEAP)."
|
"Advances in bactofection have so far been mostly incremental, but the Purdue team’s approach of combining microbiology with nanotechnology could be a big step forward" comments Guido Dietrich ("Bacteria give nanoparticles a ride"). "As well as having scientific potential, the microbots also show what can be achieved when scientists from different disciplines get together and the Purdue microbiology/nanotechnology work should lead the way for other interdisciplinary research projects."
|
|
| Bashir says that their future studies will concentrate on the development of an attenuated Listeria strain, microbot-mediated delivery of artificial biohybrid nanostructures, delivery of larger size particles and functional proteins, and investigation of solid organ tumor penetration by microbots for applications in diagnostics and therapy at the single cell level and up to a few cells. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
