| Posted: Dec 13, 2011 | |
Fighting Alzheimer's disease with nanotechnology |
|
| (Nanowerk Spotlight) Alzheimer's disease is among the most common brain disorders affecting the elderly population the world over, and is projected to become a major health problem with grave socio-economic implications in the coming decades. The total number of people afflicted by Alzheimer's disease (AD) worldwide today is about 15 million people, a number expected to grow by four times by 20501. This review looks at some of the nanotechnology-enabled approaches that are being developed for early detection and accurate diagnosis of Alzheimer's, its therapeutic treatment, and prevention. These potential solutions offered by nanotechnology exemplify the growing significance that it holds for dealing with brain ailments in general. | |
 |
|
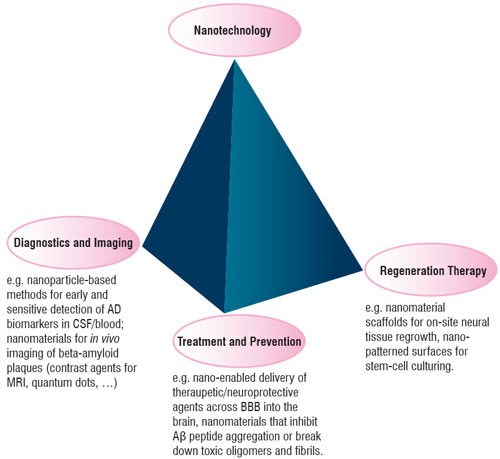
| Figure 1: Some of the applications of nanomaterials that are coming up in the context of Alzheimer's disease. | |
Diagnosing Alzheimer's disease: Nanotechnology-based developments |
|
| The pathogenesis of AD is characterized by loss of neurons and synapses, resulting in gross atrophy across multiple brain regions. The disease is presently hard to reliably diagnose, particularly in its early stages when it is often mistaken for more normal age-related or stress-related changes. While an accurate diagnosis can only be obtained post-mortem (via examination of brain tissue in an autopsy), current clinical methods involving brain imaging and neuropsychological testing are only about 85% accurate, and that too only at later stages2. | |
| Early detection of a disease is important as it can determine how efficacious any treatment would be, and also serve towards the evaluation of experimental treatments. Clinical symptoms of AD (in the form of cognitive/memory decline) usually appear much after the neural tissue begins to deteriorate. Therefore, reliable and sensitive molecular approaches are needed to detect the onset of the neurodegeneration underlying AD as early as possible. | |
| The soluble oligomers referred to as ADDLs, which have been implicated in the pathogenesis of AD, are being recognized as a potentially reliable biomarker to indicate the presence of AD, showing significant difference in Cerebrospinal fluid (CSF) concentration between AD-diagnosed subjects and age-matched healthy controls3. The typically very low concentration of such biomarkers presents too weak a signal to be reliably detected by conventional enzyme-linked immunosorbent assays. But a recently developed ultra-sensitive method, known as bio-barcode assay, shows the potential to do just that4. This technique makes use of gold nanoparticles (NPs) and magnetic microparticles suspended in solution, both of which are conjugated with an antibody specific to ADDLs while each gold NP is also attached to a large number of "barcode" DNA strands. | |
| Using this novel assay, elevated concentration of ADDLs was demonstrated in the CSF of AD cases compared with healthy subjects. The possibility of adapting the bio-barcode amplification method to blood samples rather than harder-to-obtain CSF is also being investigated5, which would help to simplify this promising diagnostic tool. | |
| Another potential diagnostic approach targeting the ADDL biomarker makes use of an optical property possessed by nanoparticles of noble metals like gold and silver called localized surface plasmon resonance (LSPR)6, 7. Systematic analysis of an assay imvolving triangular silver NPs (prepared by nanosphere lithography) employing UV-visible spectroscopy showed detectable shifts in the maximum extinction wavelength on addition of ADDLs (even as low as nanomolar concentrations) via changes in the refractive index, as well as significantly different responses to brain extracts and CSF samples from diseased patients and from controls7. | |
| Apart from these two examples, there is a number of other studies that consider nanomaterials for use in novel imaging methods that can provide in vivo means to detect, or study, pathological markers of the disease, particularly the amyloid plaques. Such imaging techniques could even serve to evaluate the clinical efficacy of new drugs designed to clear the toxic plaques. | |
 |
|
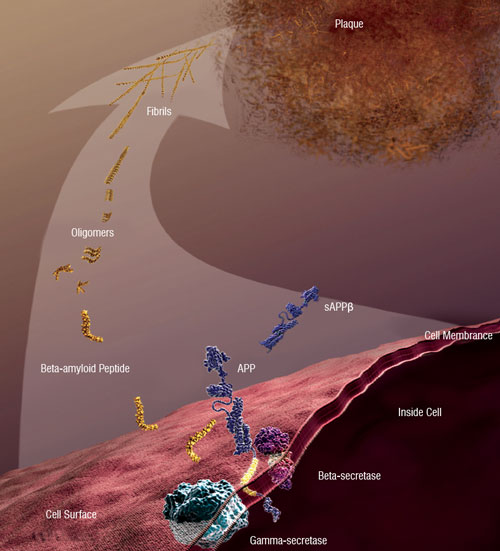
| Figure 2: Beta-amyloid peptide and AD: Abnormally accumulated amyloid-beta peptide (which is derived from the amyloid precursor protein) forms soluble oligomers and extracellular fibrillar plaques, whose deleterious effects are implicated in the neural degradation associated with AD [Image courtesy: National Institute on Aging/National Institutes of Health] | |
Potential Nano-Enabled Routes to Therapy for AD |
|
| Presently, no cure exists that would reverse the neurodegeneration caused by the advancement of AD. Medication available in the market today is mostly intended only for symptomatic benefit, for example to improve the disrupted communication between brain cells, but cannot stop the process of degeneration. Research efforts are, however, underway to develop "disease-modifying" strategies, i.e. those which can get to the root causes of AD with the aim of slowing down or halting the progressive degradation of the brain8. | |
| A major impediment stems from the existence of the so-called Blood-brain barrier (BBB) that presents a hurdle to the effective transport of theraupetic drugs/agents into the brain through the bloodstream9. The BBB, which is essentially a two-way selective filter between the blood capillaries and neural tissue, performs the beneficial function of keeping "foreign substances" like disease-causing agents and toxins circulating in the blood from getting into the brain, but, at the same time, also stops the transport of most large active molecules, even those of possibly therapeutic value, into brain tissue. Thus, strategies are needed to overcome this natural physiological barrier. | |
| In this context, various possibilities of designing nano-scale "carriers" endowed with desirable surface properties are being investigated to facilitate the efficient, targeted and safe delivery of active molecules across the BBB and their sustained release in the brain. By masking the physicochemical characteristics of the embedded/encapsulated therapeutic agents, these nanosystems could act as "trojan horses", providing a means to get such molecules across the BBB. Moreover, by shielding the encapsulated molecules against enzymatic degradation and thus enhancing their half-life, such nanocarriers could increase their bioavailability in the brain. This would naturally enhance their efficacy, and with lesser quantities of the therapeutic agent being required, any possible undesirable side effects at other sites would be minimized as well. | |
| In what follows, we present a representative sample of investigations on such nanoparticulate systems, besides other nano-related approaches, having a direct bearing on tackling AD. | |
| Nano-Mediated Drug Delivery | |
| Rivastigmine is among the few FDA-approved drugs currently in use for symptom alleviation in mild-to-moderate dementia cases. Clinically, the limited ability of the free compound to cross the blood-brain barrier, and its side effects on peripheral organs, keep it from reaching its full therapeutic potential. It is thus a suitable candidate for nanoparticle-mediated brain drug delivery. The use of biodegradable polymeric nanoparticles has recently been investigated for this purpose 10. Intravenously injected rivastigmine which was bound to Poly(n-butylcyanoacrylate) (PnBCA) nanoparticles coated with the chemical polysorbate 80 was found to have a significantly better uptake by the brain compared to the free drug (an enhancement of up to 3.82 fold was found). This enhanced delivery is explained in terms of a mechanism which involves the binding of lipoproteins present in the blood to the nanoparticle surface11. A similar positive effect was reported by another group12 in a pharmacodynamic study on amnesic mice; it was found that the use of poly(lactide-co-glycolide) (PLGA) and PnBCA NPs as carriers for rivastigmine was associated with faster reversal of memory loss compared to the free drug, indicating a more rapid and higher extent of delivery into the mouse brain. | |
| Recently, an approach to deliver the neurotransmitter acetylcholine (ACh) to the brain to remedy the disrupted cholinergic neurotransmission in AD has been proposed based on the use of single-wall carbon nanotubes (SWCNTs) as the carrier13. (It is pointed out that in this case transport to the brain was via the olfactory nerve axons rather than across the BBB). Concerns have been raised earlier over the biosafety of SWCNTs, and especially their toxicity towards the mitochondria in cells. But the present study demonstrates that by carefully controlling the dosages, it is possible to safely deliver ACh preferentially to the target organelle while at the same time avoiding its mitochondrial toxicity. In this context we also mention that the functionalization of carbon nanotubes with biomolecules such as branched Polyethylene-glycol (PEG) chains may offer a means of enhancing their biocompatibility and suitability as drug delivery vehicles14. | |
| Nano-Enabled Neuroprotective Approaches | |
| – Antioxidants | |
| An increase in the presence of free radicals due to oxidative stress can play a causative role in triggering and aggravating neural degeneration. Amyloid beta-induced oxidative stress has been implicated as a contributing factor in the neurodegeneration observed in AD15. Amyloid deposition has been found to cause dysfunction of mitochondria resulting in free radical generation, which in turn can initiate a pathway leading to apoptotic cell death16. Nanocarriers are thus also being considered for the efficient transport of anti-oxidant compounds into the brain that can neutralize such free radicals and thereby provide protection against their damaging effects, at work in diseases like AD. | |
| – Curcumin | |
| The curry spice turmeric plays a central role in the ayurvedic treatment of a wide range of ailments. The ingredient curcumin endows turmeric with many medicinal properties which have been established quite extensively in recent times43 (see Nanowerk Spotlight: "Nanotechnology-enhanced curcumin: Symbiosis of ancient wisdom of the East with modern medical science"). In the context of AD pathology, in vitro as well as animal model studies demonstrate that curcumin can have beneficial effects through multiple pathways, such as its strong anti-oxidant activity17, 18, interaction with metal ions and preventing metal-induced aggregation of beta-amyloid19, 20 and destabilizing preformed beta-amyloid fibrils as well as inhibiting Aβ aggregate formation21-23. Thus, administration of curcumin is being seen as a potent theraupetic (and preventive) strategy against AD24. | |
| – Fullerene-based Neuroprotection | |
| The well-known buckminsterfullerene (C60) has been referred to as a "free radical sponge" owing to its potent anti-oxidant activity25, but native C60 is soluble only in a limited number of biologically unattractive solvents, such as toluene and benzene. As a biologically more suitable alternative, water-soluble carboxylic acid-functionalized forms of C60 (carboxyfullerenes) have been investigated for their neuroprotective potential against free radicals. In a study on cultured cortical neurons26, the malonic acid derivative of C60 was found to be able to eliminate both the superoxide anion and the hydroxyl radical, and to reduce the apoptotic neuronal death induced by exposure to the AD amyloid-beta(1-42) peptide. Water-soluble hydroxyl derivatives of fullerene, known as fullerenols, are another species whose ability to scavenge free radicals and exert a neuroprotective effect has been demonstrated in cortical cell culture studies27, 28. | |
| – Chelation Therapy | |
| Mounting evidence makes a case for a link between abnormal concentration of certain metals in the brain and the neurodegeneration associated with AD. The accumulation of redox-active metal ions like iron can be a source of toxic free radicals contributing to oxidative damage29, 30. ROS-generating Aβ-metal redox reactions have been recently hypothesized as a key mechanism for the neurotoxicity of beta amyloid oligomers. A progressive accumulation of metals has been observed in the AD brain during disease progression from moderate to severe AD31. In vitro observations suggest that biometals may bind to Aβ peptide and promote its aggregation and formation of insoluble deposits32-35, a possibility consistent with the enriched levels of metals like Cu, Zn and Fe that have been observed in senile plaques compared to the healthy brain36. | |
| Given these and other similar results, the use of metal-chelating compounds to correct the imbalance of metals in the brain and attenuate metal-mediated toxic effects is being recognized as a promising approach to neuroprotection as well as therapeutic intervention with regard to AD37. There is, however, the question of how such chelating agents may be translocated to the brain and the resulting metal-chelator conjugates efficiently expelled if required, besides the possibility of cytotoxic effects of the free compounds. This has led to studies on nanoparticle-mediated delivery for overcoming the inherent limitations of metal-chelating agents for treating the brain. | |
| Direct Interaction of Nanostructures with the Amyloid-β Peptide | |
| The use of nanoparticulate formulations for confronting AD is not limited merely to carrying drugs and other therapeutic compounds across the BBB and delivering them to the brain. Several studies are also aimed at designing brain-specific NPs with an affinity for beta-amyloid peptides. Such nanomaterials could directly interact with the peptide (e.g. through adsorption of the latter onto the surface of the NPs which could alter the peptide conformation) and thereby either suppress its self-assembly into toxic oligomers and fibrillar plaques, or mediate the breakdown of already existing amyloid aggregates and thus eliminate their associated deleterious effects. A few illustrative examples: | |
| – PEGylated phospholipid nanomicelles were found to inhibit Aβ peptide self-assembly by inducing a conformational change that makes the peptide averse to aggregation, and a lowered neurotoxicity was demonstrated in cytotoxicity studies on human neuroblastoma cells38. A similar ability to interact with the amyloid-beta peptide and inhibit its fibril formation has been seen with fluorinated nanoparticles (complexes of polyampholyte and fluorinated dodecanoic acid)39. NPs synthesized by sulfonation and sulfation of polystyrene also show such an inhibitory activity by affecting the conformation of the adsorbed amyloid-beta peptide and inducing an unordered state40. | |
| – Biocompatible cholesterol-bearing pullulan (CHP)-based nanogels have been suggested for use as artificial chaperones (proteins which influence the folding of other proteins). An ex vivo study showed that such nanogels (with diameter of 20-30 nm) could incorporate6-8 Aβ molecules each and induce a conformation change in the peptide, thereby preventing its aggregation and inhibiting amyloid fibrils from forming41. | |
| – Quantum dot nanocrystals (QDs), which we have encountered earlier in the context of imaging applications, have also been found to be effective in decreasing amyloid fibril formation. These studies have made use of QDs capped with suitable organic compounds (N-acetyl-L-cysteine42 and Dihydrolipoeic acid43), and indicate that fairly low concentrations of the QDs (compared with the peptide concentration) suffice in showing the inhibitory effect. | |
| – An interesting technique has been recently proposed to remotely and selectively dissolve fibrillar amyloid deposits by means of concentrated thermal energy, which is generated by a combination of weak microwave fields and gold NPs already attached to the amyloid-β target44, 45. The microwave energy source is about 6 times weaker than that present in conventional mobile phones, and gold NPs were selected here due to their high surface-to-volume ratio, biocompatibility and high mobility. This approach is, however, associated with the possibility of forming oligomers following breakdown of the fibrillar species, which are neurotoxic as well. Thus, some improvement is needed to make this strategy safer and more effective, perhaps through a modification whereby the smaller oligomeric species themselves are targeted for disaggregation. | |
| Towards Regenerative Therapy | |
| The central nervous system has little capacity for natural regeneration, and the gradual loss of brain cells caused by the toxic amyloid species is thus irreversible. This limits the potential of medical therapies which target amyloid aggregation and its toxicity to only slowing or stopping the degeneration of neural tissue in AD cases. However, ongoing work on some novel approaches, such as those based on stem cells and tissue engineering, offers a glimmer of hope for reversing the disease-induced neural loss and restoring normal brain function46-48. Nanotechnological applications are beginning to emerge in this field too49-51, (e.g. the development of nanoengineered scaffolds that can mimic the extracellular matrix to support and promote the growth and controlled differentiation of neural progenitor cells in vivo for repairing damage). | |
| Here we mention one recent development of possible relevance to AD. The current approach to adult stem cell therapy typically requires a small number of stem cells to be harvested from the patient, and then grown in the laboratory to create a batch of sufficient volume which can initiate the process of tissue regeneration on re-injection into the body. A difficulty with this culturing process on standard plastic surfaces is the tendency of stem cells in culture to spontaneously differentiate into other cells and thus lose their innate regenerative capacity. A paper published earlier this year51, 52 reported on the use of a novel surface patterned with an ordered arrangement of nanoscale pits (manufactured with electron beam lithography + injection moulding), which was shown to be much more effective in allowing stem cells to proliferate while also maintaining their stem cell characteristics over a long period, for use in therapy. Such nano-patterned surfaces, the research team suggests, may provide a basis for large-scale stem cell culture "factories" enabling therapies for various diseases, including AD and Parkinson's. | |
Recapitulation and Future Directions |
|
| Nanotechnology offers the potential for engineering novel nano-scale materials and devices with controllable functional properties. In the context of treating Alzheimer's disease, such nanoconstructs could, on one hand, provide the means to carry and deliver medication as well as other therapeutic/neuroprotective molecules efficiently to the brain, and on the other, they could also be tailored to more directly take on and eliminate the pathogenic factors underlying the disorder (such as the abnormal protein aggregation into oligomers and fibrils). Nanomaterials are also beginning to be used as a basis for approaching the important challenge of sensitive and early AD detection. | |
| The selection of R&D work presented above has been intended to convey a sense of the growing role that nanoscience and technology are set to play in the ongoing global effort to develop diagnostics and treatments for neurological disorders (Fig. 1). As many of the promising results described above have been obtained in laboratory in vitro studies, it is important to confirm the efficacy of such nano-enabled approaches in representative in vivo animal models of AD. | |
| Besides this, there is the question of inadvertent side effects to be kept in mind, just as it needs to be when evaluating any nano-based application, medical or otherwise, with direct human relevance. In the very complex biochemical environment of the central nervous system in particular, the possibility of the administered nanoscale systems, especially non-biodegradable ones, having unwanted cytotoxic consequences (apart from the intended function) cannot be ignored. Thus, their overall safety and biocompatibility need to be thoroughly assessed before any such application comes up for clinical trials. | |
| References | |
| 1. R Rookmeyer et al., "Forecasting the Global Burden of Alzheimer's Disease", Alzheimer's and Dementia, 3 (2007) 186-191 | |
| 2. D.S. Knopman et al., "Practice Parameter: Diagnosis of Dementia (An Evidence-based Review)", Neurology, 56 (2001) 143-1153 | |
| 3. C.D. Keating, "Nanoscience Enables Ultrasensitive Detection of Alzheimer's Biomarker", Proc Natl Acad Sci., 102 (2005) 2263-2264 | |
| 4. D. G. Georganopoulou et al., "Nanoparticle-based Detection in Cerebral Spinal Fluid of a Soluble Pathogenic Biomarker for Alzheimer's Disease", Proc. Natl Acad Sci., 102 (2005) 2273-2276 | |
| 5. D.A. Davis, W. Klein and L. Chang, "Nanotechnology-based Approaches to Alzheimer's Clinical Diagnostics", Nanoscape, 3 (2006) 13-17 | |
| 6. A.J. Haes et al., "A Localized Surface Plasmon Resonance Biosensor: First Steps Toward an Assay for Alzheimer's Disease", Nano Lett., 4 (2004) 1029-1034 | |
| 7. A.J. Haes et al., "Detection of a Biomarker for Alzheimer's Disease from Synthetic and Clinical Samples Using a Nanoscale Optical Biosensor", J Am Chem Soc., 127 (2005) 2264-2271 | |
| 8. D. Galimberti and E. Scarpini, "Disease-modifying Treatments for Alzheimer's Disease", Therapeutic Advances in Neurological Disorders, 4 (2011) 203-216 | |
| 9. M. Simko et al., "Can nanoparticles End up in the Brain?", NanoTrust-Dossier, 014en (2010) | |
| 10. B. Wilson et al., "Poly(n-butylcyanoacrylate) Nanoparticles Coated with Polysorbate 80 for the Targeted Delivery of Rivastigmine into the Brain to Treat Alzheimer's Disease", Brain Res., 1200 (2008) 159-168 | |
| 11. J. Kreuter et al., "Apolipoprotein-Mediated Transport of Nanoparticle-Bound Drugs Across the Blood-brain Barrier", J Drug Target., 10 (2002) 317-325 | |
| 12. S.A. Joshi, S.S. Cavhan and K.K. Sawant, "Rivastigmine-loaded PLGA and PBCA Nanoparticles: Preparation, Optimization, Characterization, in Vitro and Pharmacodynamic Studies", Eur J Pharm Biopharm., 76 (2010) 189-199 | |
| 13. Z. Yang et al., "Pharmacological and Toxicological Target Organelles and Safe use of Single-walled Carbon nanotubes as Drug Carriers in Treating Alzheimer's Disease", Nanomed Nanotechnol Biol Med., 6 (2010) 427-441 | |
| 14. Z. Liu et al., Circulation and Long-term Fate of Functionalized, Biocompatible Single-walled Carbon nanotubes in Mice Probed by Raman Spectroscopy", Proc Natl Acad Sci., 105 (2008) 1410-1415 | |
| 15. D.A. Butterfield, "Amyloid Beta-peptide (1-42)-induced Oxidative Stress and Neurotoxicity: Implications for Neurodegeneration in Alzheimer's Disease Brain. A Review", Free Radic Res., 36 (2002) 1307-1313 | |
| 16. H. Kadowaki et al., "Amyloid β Induces Neuronal Cell Death through ROS-mediated ASK1 Activation", Cell Death Diff., 12 (2005) 19-24 | |
| 17. Y.R. Mahajan, "Nanotechnology - Enhanced Curcumin: Symbiosis of Ancient Wisdom of East with Modern Medical Science", Nanotech Insights, 2 (2011) 17-27 | |
| 18. J.M. Ringman et al., "A Potential Role of the Curry Spice Curcumin in Alzheimer's Disease", Curr Alzheimer Res., 2 (2005) 131-136 | |
| 19. S. Daniel et al., "Through Metal Binding, Curcumin Protects against Lead- and Cadmium-induced Lipid Peroxidation in Rat Brain Homogenates and against Lead-induced Tissue Damage in Rat Brain", J Inorg Biochem., 98 (2004) 266-275 | |
| 20. L. Baum and A. Ng, "Curcumin Interaction with Copper and Iron Suggests One Possible Mechanism of Action in Alzheimer's Disease Animal Models", Alzheimer's Dis., 6 (2004) 367-377 | |
| 21. F. Yang et al., "Curcumin Inhibits Formation of Amyloidbeta Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid In Vivo", J Biol Chem., 280 (2005) 5892-5901 | |
| 22. M. Garcia-Alloza et al., "Curcumin Labels Amyloid Pathology in vivo, Disrupts Existing Plaques and Partially Restores Distorted Neurites in an Alzheimer Mouse Model", J Neurochem., 102 (2007) 1095-1104 | |
| 23. K. Ono et al., "Curcumin has Potent Anti-amyloidogenic Effects for Alzheimer's Beta Fibrils In Vitro", Neurosci Res., 75 (2004) 742-750 | |
| 24. S. Mishra and K. Palanivelu, "The Effect of Curcumin (turmeric) on Alzheimer's Disease: An Overview", Ann Indian Acad Neurol., 1(2008) 13-19 | |
| 25. P.J. Krusic et al., "Radical Reactions of C60", Science, 254 (1991) 1183-1185 | |
| 26. L.L. Dugan et al., "Carboxyfullerenes as Neuroprotective Agents", Proc. Natl. Acad. Sci. USA., 94 (1997) 9434-9439 | |
| 27. L.L. Dugan et al., "Buckminsterfullerenol Free Radical Scavengers Reduce Excitotoxic and Apoptotic Death of Cultured Cortical Neurons", Neurobiol Dis., 3 (1996) 129-135 | |
| 28. H.M. Huang et al., "Blockage of Amyloid β Peptide-Induced Cystolic Free Calcium by Fullerenol-1, Carboxylate C60 in PC 12 Cells", Life. Sci., 66 (2000) 1525-1533 | |
| 29. X. Huang et al., "Redox-Active Metals Oxidative Stress and Alzheimer's Disease Pathology", Ann N Y Acad. Sci., 1012 (2004) 153-163 | |
| 30. M.A. Smith et al., "Iron Accumulation in Alzheimer Disease is a Source of Redox-Generated Free Radicals, Proc. Natl. Acad. Sci .USA., 94 (1997) 9866-9868 | |
| 31. K.S.J. Rao et al., "Trace Elements in Alzheimer's Disease Brain: A New Hypothesis", Alz Rep., 2 (1999) 241-246 | |
| 32. A.I. Bush et al., "Rapid Induction of Alzheimer Abeta Amyloid Formation by Zinc", Science., 265 (1994) 1464-1467 | |
| 33. X. Huang et al., "Zinc-Induced Alzheimer's Abeta 1-40 Aggregation is Mediated by Conformational Factors", J. Biol. Chem., 272 (1997) 26464-26470 | |
| 34. P. Faller, "Copper and Zinc Binding to Amyloid-Beta: Coordination, Dynamics, Aggregation, Reactivity and Metal-Ion Transfer", Chem. Biochem., 10 (2009) 2837-2845 | |
| 35. X. Huang et al., "Trace Metal Contamination Initiates the Apparent Auto-Aggregation, Amyloidosis and Oligomerization of Alzheimer's Abeta Peptides", J. Biol. Inorg. Chem., 9 (2004) 954-960 | |
| 36. M.A. Lovell et al., "Copper, Iron and Zinc in Alzheimer's Disease Senile Plaques", J Neurol Sci., 158 (1998)47-52 | |
| 37. M.L. Hegde et al., "Challenges Associated with Metal Chelation Therapy in Alzheimer's Disease", J Alzheimers Dis., 17 (2009) 457-468 | |
| 38. A.S. Pai, I. Rubinstein and H. Onyuksel, "PEGylated Phospholipid Nanomicelles Interact with Beta-Amyloid(1-42) and Mitigate its Beta-Sheet Formation, Aggregation and Neurotoxicity In vitro, Peptides, 27 (2006) 2858-2866 | |
| 39. S. Rocha et al., "Influence of Fluorinated and Hydrogenated Nanoparticles on the Structure and Fibrillogenesis of Amyloid Beta-Peptide", Biophys Chem., 137 (2008) 35-42 | |
| 40. A.M. Saraiva et al., "Randomization of Amyloid-β Peptide(1-42) Conformation by Sulfonated and Sulfated Nanoparticles Reduces Aggregation and Cytotoxicity", Macromol Biosci., 10 (2010) 1152-1163 | |
| 41. K. Ikeda et al., "Inhibition of Amyloid β Peptide Fibril Formation by Hydrogel Nanoparticles", Pept Sci., 2005 (2006) 313-316 | |
| 42. L. Xiao et al., Inhibition of Beta 1-40 Amyloid Fibrillation with comN-Acetyl-L-Cysteine Capped Quantum Dots, Biomaterials, 31 (2010) 91-98 | |
| 43. G. Thakur et al., "Conjugated Quantum Dots Inhibit the Amyloid β (1-42) Fibrillation Process", Int J Alzheimers Dis., 2011 (2011) 502386 | |
| 44. N. Bastus et al., Gold Nanoparticles for Selective and Remote Heating of β-Amyloid Protein Aggregates", Mater Sci Eng., C 27 (2001) 1236-1240 | |
| 45. M.J. Kogan et al., "Nanoparticle-Mediated Local and Remote Manipulation of Protein Aggregation", Nano Lett., 6 (2006) 110-115 | |
| 46. T.R. Yamasaki et al., "Neural Stem Cells Improve Memory in an Inducible Mouse Model of Neuronal Loss", J Neurosci., 27 (2007) 11925-11933 | |
| 47. J.H. Kim et al., "Dopamine Neurons Derived from Embryonic Stem Cells Function in an Animal Model of Parkinson's Disease", Nature, 418 (2001) 50-56 | |
| 48. U. Englund et al., "Grafted Neural Stem Cells Develop into Functional Pyramidal Neurons and Integrate into Host Cortical Circuitry", Proc Natl Acad Sci USA., 99 (2002) 17089-17094 | |
| 49. T.R. Nayak et al., "Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells", ACS Nano, 5 (2011) 4670-4678 | |
| 50. G.A. Silva et al., "Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers", Science 303(2004) 1352-1355 | |
| 51. R.J. McMurray et al., "Nanoscale Surfaces for the Long-Term Maintenance of Mesenchymal Stem Cell Phenotype and Multipotency", Nature Materials, 10 (2011) 637-644 | |
| 52. doi:10.1038/nmiddleeast.2011.100 | |
| By Gaurang Mahajan ([email protected]), Hyderabad, India. This Nanowerk Spotlight is an excerpted version of an article that will be published in the forthcoming October 2011 Issue of Nanotech Insights. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
