| Posted: Jan 04, 2012 | |
Nitric oxide releasing nanoparticles for effective treatment of infections |
|
| (Nanowerk Spotlight) Nitric oxide (NO) is known to possess impressively broad antimicrobial activity due to both its inherent ability to inhibit growth and kill pathogens as well as its function as a potent immunostimulatory signaling molecule. Research data shows that NO is a potentially powerful therapeutic for serious skin and soft-tissue infections, including MRSA (methicillin-resistant S. aureus) infected wounds. However, as a highly reactive gas, NO has proven difficult to deliver in a convenient and cost effective therapeutic format. This limitation has largely precluded its routine use, even in hospital settings. | |
| In new work, researchers have now demonstrated the potential application of NO as an antimicrobial agent in the setting of skin and soft tissue infections. | |
| "The nitric oxide releasing nanoparticles presented in our paper are novel sustained slow release NO donors without many of the shortcomings of other NO-donors such as organic nitrates, the most commonly used NO-donor in clinical practice" Adam Friedman, Director of Dermatologic Research and Associate Residency Program Director at the Unified Division of Dermatology of Albert Einstein College of Medicine, tells Nanowerk. | |
| These new findings are in concert with the team's previous investigations evaluating the efficacy of NO-releasing nanoparticles (NO-nps) in the treatment of skin and soft tissue infections, including dermal abscesses. This study takes the next step to evaluate the ultimate reach of this technology with even deeper infections, intramuscular abscesses or pyomyositis. In addition, unlike in previous studies, the scientists compared the NO-nps to a well established antibacterial agent, vancomycin, which was not as effective as the NO-nps in eradicating the bacterial burden and clearing the infection. | |
| Friedman and his team report their findings in the January/February 2012 edition of Virulence ("Nitric oxide nanoparticles: Pre-clinical utility as a therapeutic for intramuscular abscesses"). | |
 |
|
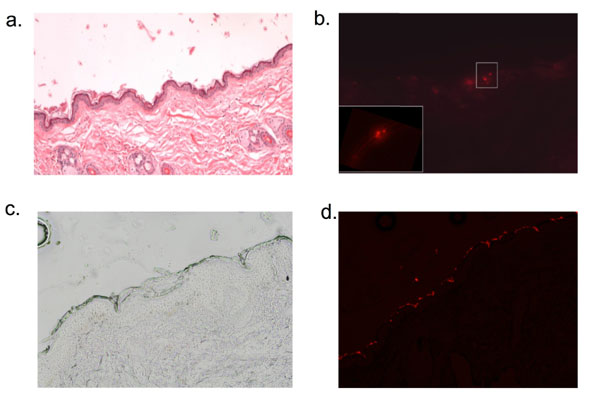
| NO-nps penetrate rat skin (a-b). Fluorescently labeled nanoparticles were applied to the abdomen of of Sprague-Dawley rats under occlusion for 30 minutes, following which time the skin was cleaned thoroughly with an alcohol wipe. 4mm punch biopsy was take from the site of application, and representative sections are shown. H&E (a.) staining shows normal epidermal and dermal architecture and fluorescent imaging (b.) demonstrates labeled particles within the epidermis in the spinous layer ( Magnification 10x; inset 40x). NO-nps penetrate human skin ex vivo (c-d). Fluorescently labeled nanoparticles were applied to the surface of discarded human back skin (following mohs surgery) for thirty minutes. Tissue was kept in a PBS water bath during the application time of 30 minutes. Frozen sections were taken from the specimens after being wiped with alcohol and light microscopy (c.) and fluorescent imaging (d.) was performed. Again, particles were visualized in the epidermis. (Image: Dr. Adam Friedman, Albert Einstein College of Medicine) | |
| Friedman explains that the main limitation of organic nitrates is decreased efficacy with prolonged continuous use – a so-called 'nitrate tolerance' – resulting from depletion of tissue thiols, an induced "de-sensitivity' of soluble Guanylyl Cycylase (sGC) to NO, or increase in breakdown of cyclic GMP (the product of NO binding to sGC) by phosphodiesterase. | |
| Unlike nitrites/nitrates, nitric oxide releasing nanoparticles developed by Friedman and his collaborators do not require an external reducing agent. | |
| "The NO is generated within the nanoparticle through a novel thermal reduction scheme, and therefore bypasses these limitations," says Friedman. "The NO-np combine alkoxysilanes based hydrogels, which provide a robust skeleton with a network of interweaving pores or channels, with a polysaccharide derived glassy matrix which serves two unique functions: One, it facilitates the internal redox chemistry that allows for the facile glucose-mediated conversion of nitrite to NO; and two, it keeps the pores 'plugged' as long as the particles are dry but begins to 'melt' when exposed to water, thereby allowing escape of the NO." | |
| Furthermore, simple manipulation of pore size by altering components of the nanoparticle protocol, such as the molecular weights of polyethylene glycol used, has been shown to predictably alter release of the nanoparticle payload. The ease with which NO release can be modulated can allow for the generation of clinical target-specific nanoparticles in which either slow or immediate release of NO is warranted. | |
| The potential of the nitric oxide nanoparticles, as is once again highlighted by this paper, shows that both topical and intralesional administration effectively clears intramuscular MRSA infections. Furthermore, the research team believes that this technology not only has great potential as a therapeutic agent for the treatment of skin and soft tissue infections but may also as a promising tool to promote the understanding of NO signaling mechanisms – a platform that can allow for systematic study of how locally produced NO and related molecules impact infection and wound healing. | |
| "The role of this technology in accelerating wound healing has been demonstrated via in vitro fibroblast migration assays with PCR analysis of collagen gene expression, and in an in vivo splinted as well as immunocompromised NOD-SCID murine wound model, demonstrating the clear importance of NO in wound closure," says Friedman. | |
| Elaborating on this statement, he points out that the potential for broad applicability for this nanoparticulate platform is emerging though a series of translational collaborations: Broad spectrum antimicrobial in vitro efficacy was demonstrated against resistant clinical isolates of Gram Positive1, 2 Gram Negative1, 3 and candidal clinical species. Topical application of NO nanoparticles to in vivo MRSA and A. baumannii infected excision models results in acceleration of wound healing and clearance of bacterial burden as compared to controls clinically and histologically2, 3. To extend these results further, topical application of NO-nps in an induced in vivo MRSA abscesses model was investigated, demonstrating a dose dependent impact on lesion resolution based on wound size, histology, and cytokine profiling from the infected sites4. | |
| "In our current study, topical and intralesional treatment with NO-nps in the MRSA intramuscular abscess model was shown to be significantly more effective than Vancomycin based on clinical assessment, wound cultures, and histopathology," says Friedman. "This is the first head to head in vivo assay comparing the NO releasing nanotechnology to a gold standard clinical therapy, further highlighting the benefit of utilizing nanotechnology in this arena." | |
| He notes that, beyond the antimicrobial and wound healing applications, the important role of nitric oxide in maintaining vascular health also lead to the team's testing of the efficacy of NO nanoparticles in addressing conditions associated with endothelial dysfunctions: "NO nanoparticles were shown to increase erectile function when applied topically to the penis of rats that were developed as a model of erectile dysfunction5, and even more importantly, in a post-radical prostatectomy animal model with cavernous nerve transection – a clinical scenario in which phosphodiesterase 5 inhibitors (i.e. Viagra) are ineffective." | |
| He explains that, in a dose-dependent manner, intravenously (IV) administered, circulating NO-nps increased exhaled NO concentrations, decreased mean arterial blood pressure (MAP) and increased microvascular flow over several hours, without inducing an inflammatory response as compared to control nanoparticles6. | |
| "When compared to two well known NO donors, DETA NONOate and DPTA NONOate, similar decreases in MAP were witnessed" says Friedman. "However, the impact on vascular tone following NONOate use was highly inefficient as compared to NO nanoparticles, requiring 30 times more NO release to induce a similar physiological response. Additionally, relative to the NO-nps, there is enhanced methemoglobin formation by NONOate administration with subsequent decrease in hemoglobin oxygen carrying capacity. IV NO nanoparticles are observed both to counteract systemic hypertension following infusion of an NO scavenging hemoglobin based oxygen carrier7, improving systemic and microvascular function, and to correct negative hemodynamic changes during hemorrhagic shock8. These data suggests that the NO nanoparticles have a clear potential to replenish NO in situations were NO production is impaired, insufficient or consumed (e.g. endothelial dysfunction, metabolic disorders and hemolytic diseases)." | |
| It therefore appears that the clinical translation of this technology is potentially broad and far reaching to many fields in Medicine and Surgery. | |
| The immediate next step for the researchers is to file an Investigational New Drug (IND) Application with the U.S. Food and Drug Administration (FDA) in order to begin phase I trials. | |
| "We anticipate that the initial indication will be for simple skin infections, with the hopes of extending these studies to those involving more recalcitrant skin and soft tissue infections" says Friedman. "As indicated by our pre-clinical work, there are many potential routes of application ranging from topical, intralesional to intravenous, thereby expanding the range of clinical applications." | |
| According to the team, the greatest challenge facing the development and translation to the bedside is two-fold but intertwined: First, the rigorous and lengthy clinical evaluation of the technology as mandated by the FDA; and second, the cost of these investigations: "In this time of economic turmoil and downturn, it is difficult to acquire the funds necessary to fully develop a new product, whether they be from federal (NIH) or private (venture capital, private investors) sources" notes Friedman. "Fortunately, we are progressing forward with the collaboration and expertise of Makefield Therapeutics Inc, and are optimistic that this technology will ultimately reach and help its intended target, the patient." | |
| Additional References | |
| 1. Friedman A, Blecher, K, Sanchez, D, Tuckman-Vernon, C, Gialanella, P, Friedman, JM, Martinez, LR, and Nosanchuk, JD. Susceptibility of Gram Positive and Negative Bacteria to Novel Nitric Oxide-Releasing Nanoparticle Technology. Virulence. 2011;2(3). | |
| 2. Martinez LR, Han G, Chacko M, et al. Antimicrobial and Healing Efficacy of Sustained Release Nitric Oxide Nanoparticles Against Staphylococcus Aureus Skin Infection. Journal of Investigative Dermatology. Oct 2009;129(10):2463-2469. | |
| 3. Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. Nitrix oxide releasing nanoparticles are therapeutic for Acinetobacter baumanni wound infections. Virulence. 2010;1(2):62-67. | |
| 4. Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS One. 2009;4(11):e7804. | |
| 5. Han G, Tar M, Kuppam DS, et al. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med. Jan 2010;7(1 Pt 1):224-233. | |
| 6. Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long lived circulating nanoparticles. Free Radic Biol Med. May 8 2010. | |
| 7. Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. Jan 2011;300(1):H49-56. | |
| 8. Nachuraju P, Friedman AJ, Friedman JM, Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. Feb 20 2011. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
