| Posted: Feb 13, 2012 | |
Exploding microcapsules track down and kill cancer cells |
|
| (Nanowerk Spotlight) With the advent of nanomedicine, the concept of a "magic bullet" to fight cancer is getting closer to reality. Previously an idea straight out of science fiction, researchers around the world are working on perfecting nano- and microscale drug carriers that get injected into the body, transport themselves to the correct target, such as a tumor, and deliver the required dose of a medication or other substance to effectively destroy or repair this target. | |
| There are two key components of a drug carrier system in order for it to be effective: it needs to identify and reach its target; and it needs to release its payload at the target at the right time or over a longer period of time. This controlled release, however, has proven to be quite a challenging issue. Often, part of the drug are already released in the blood stream prior to reaching the target cells. | |
| "It is of great importance to design intelligent drug carriers that can specifically respond to physiopathological signals and allow explosive release of the loaded drugs while entrapping the drugs efficiently during the process of blood circulation," Xian-Zheng Zhang, Director of the Key Laboratory of Biomedical Polymers of Ministry of Education and a professor in the Department of Chemistry at Wuhan University in China, explains to Nanowerk. | |
| To avoid the side effects of prematurely released toxic cancer drugs on healthy tissues, Zhang and his team designed and fabricated an "active defense" system which could effectively keep the drug entrapped in its carrier in the blood and normal tissues whereas it would allow the explosive drug release under the right physiopathological stimuli once the drug carrier reaches the cancerous tissues. | |
| "As far as we know, developing satisfactory drug delivery systems which could explosively release the drug in response to environmental stimuli still remains a great challenge," says Zhang. "Explosion of nanoparticles for the sudden release of substance by a thermal treatment has been reported previously. However, it was not suitable for biological applications since it required a high temperature (above 100°C). Self-exploding capsules that consist of a degradable core surrounded by a semi-permeable shell were also reported. For these capsules, when the microgel core degraded, the swelling pressure caused leakage of the shell membrane to release its payload. These systems, however, were limited by the base-driven requirement, which was difficult to be satisfied in biological systems." | |
| In a previous study, Zhang's team also prepared microcapsules that could release a drug rapidly under physiological conditions upon addition of dithiothreitol (DTT). Upon the addition of DTT, the crosslinkages containing disulfide bonds are cleaved, leading to increased swelling pressure of the dextran core. When the swelling pressure is higher than the membrane can resist, the surrounding LbL membrane will rupture followed by an exploding release of the entrapped drug ("Controllable exploding microcapsules as drug carriers"). | |
| Obviously, this need of an external trigger – which needs to be injected at the tumor site – is the limitation of this system. In their new work, the researchers demonstrate an "active defense" system that allows the explosive drug release once the drug carrier is activated by the environmental stimuli present at the cancerous tissues. The team reported their findings in the February 2, 2012 online edition of Advanced Functional Materials ("Design of an 'Active Defense' System as Drug Carriers for Cancer Therapy"). | |
| To fabricate their microcapsules, the scientists first prepared biodegradable dextran microgels crosslinked by a Schiff base. Then they coated the surface of the microgels with a layer-by-layer (LbL) membrane. | |
 |
|
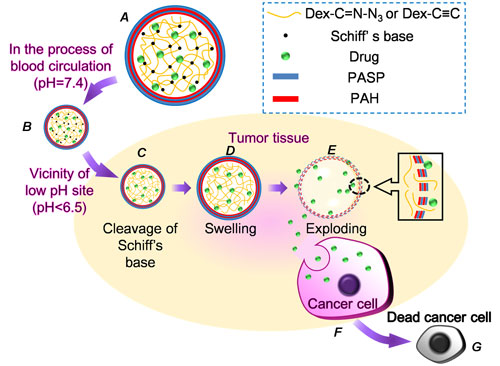
| Structure of a microcapsule and tumor triggered exploding of the microcapsule. A) Structure of microcapsules, B) microcapsules host drugs at pH 7.4, C) Schiff's base cleaves at low pH site, D) increased pressure causes the microcapsule to swell, E) exploding of the microcapsule, F) uptake of drugs released from exploding microcapsules by cancerous cell, and G) apoptosis of tumor cells. (Image: Dr. Zhang, Wuhan University) | |
| Once in blood circulation, the system is activated by the physiopathological stimuli – an acidic environment – at the cancer site: the Schiff base hydrolyzes in acidic environments. This hydrolization causes the crosslinkages containing C=N bonds to be cleaved, leading to increased swelling pressure of the dextran core. When the swelling pressure is higher than the membrane can resist, the surrounding LbL membrane will rupture followed by an explosive release of the entrapped drug, which is then uptaken by the cancer cells. | |
| Zhang cautions that the "active defense" system still has some drawbacks with regard to practical use, such as the relatively large size of microcapsules, that can be cleared from blood, and a lack of passive targeting properties. | |
| "However" he says, "these drawbacks can be overcome if we further functionalize the microcapsules, including the change of the size of microcapsules; incorporation of PEG to prolong the blood circulation period; and incorporation of functional peptide sequences such as RGD to endow with targeting properties." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
