| Posted: Mar 08, 2012 | |
Environmental implications of nanoparticle aging |
|
| (Nanowerk Spotlight) Several studies in the literature have highlighted that as nanomaterials "age" they can undergo oxidation; sintering (coalescence); surface ligand displacement; smaller nanoparticle formation; and surface carbonate formation. Nevertheless, no studies are available on how these changes affect the physicochemical properties of the nanomaterials. | |
| The aging of nanomaterials is expected to be rapid even under ambient environmental conditions. With the consequence that pristine, as synthesized materials – which are commonly used in nanotechnology-relevant environmental health and safety (EHS) studies – are never really encountered under natural environmental conditions. Which means that researchers who investigate the applications and implications of nanomaterials need to have a clear understanding of the aging process of these materials and need to take its effects into consideration. | |
| "Most, if not all, nano EHS related research is conducted using freshly synthesized or newly purchased nanomaterials" Vicki H. Grassian, F. Wendell Miller Professor in the Departments of Chemistry and Chemical and Biochemical Engineering, and Director of the Nanoscience and Nanotechnology Institute at the University of Iowa (UI), tells Nanowerk. "However a number of publications over the recent years have indicated that these nanomaterials are highly dynamic in nature and change over time as they age under ambient conditions and in the environment. This results in changes in size, morphology, composition and surface functionality. Thus there can be significant deviations from the predicted behavior from the EHS studies conducted with fresh samples." | |
| New research by Grassian's group, reported in a recent edition of Environmental Science & Technology ("Environmental Implications of Nanoparticle Aging in the Processing and Fate of Copper-Based Nanomaterials"), first-authored by Imali A. Mudunkotuwa and co-authored with Dr. John M. Pettibone, shows clear differences between the aged and new copper nanoparticle processing under same environmental conditions which can lead to differences in their fate, transformation and toxicity. | |
| The basic findings of this work is that copper-based nanoparticles age in the ambient environment and change over time. Furthermore, these aged nanoparticles show different behavior in aqueous suspensions with respect to dissolution. | |
 |
|
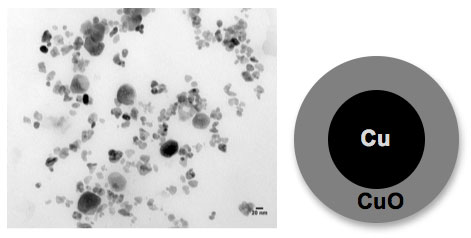
| The figure above shows a transmission electron microscopy (TEM) image of aged copper nanoparticles. These nanoparticles are oxidized giving a core shell morphology with a metallic copper (Cu) core and a fully oxidized copper oxide (CuO) shell (see cartoon representation). Additionally, there appear to be larger particles in the image from particle coalescence. These aged nanoparticles show different chemical behavior compared to new Cu nanoparticles and fully oxidized CuO nanoparticles. (Image: Imali A. Mudunkotuwa, Grassian research group, University of Iowa) | |
| "Our study highlights the important of oxidation and the thickness of the oxide layer on copper nanoparticles behavior," says Grassian. "These aged materials show different behavior when compared to metallic new (Cu) nanoparticles and fully oxidized copper oxide (CuO) nanoparticles. Although aged copper and copper oxide nanoparticles have the same surface composition the adsorption of organic acids showed distinct differences between the two samples indicating potential coupling of the surface with the underlying core." | |
| She notes that in many nano EHS studies the samples are pretreated by sonication, dispersing in buffers and biological media. These can result in conditions which can either mitigate or enhance the aging process. | |
| "In situations where the aging is enhanced, the responses obtained may arise not from the initially characterized materials but from the aged materials," says Grassian. "For example, our studies show that sonication of copper nanoparticle suspension – which is used in animal studies – increase the Cu2O fraction of the sample and therefore the amount of copper in the Cu(I) oxidation state; which can play a role in the production of reactive oxygen species. Thus when considering the toxicological responses it will be important to account for this oxidized fraction as well." | |
| Furthermore, when a study is conducted over a long period of time aging of nanomaterials can result in inconsistent data from different laboratories or even the same laboratory. Thus, it is important to characterize these materials for each study conducted. | |
| Copper and copper oxide nanoparticles have shown toxicological responses particularly in aquatic species and animal studies (see for instance: "Copper nanoparticles harm zebrafish"). That is why the UI team has undertaken a study of three different types of copper nanoparticles that differ in their level of oxidation. | |
| As Grassian points out, a comparison of the aqueous behavior between newly purchased commercially manufactured copper nanoparticles to nanoparticles that were allowed to sit in the laboratory environment for several years under ambient conditions is particularly interesting as it probes potential effects of aging on environmental processing and the fate and transport of these materials. | |
| "The aged materials exhibit differences in solubility, aggregation and reactivity that can affect the mobility and toxicity of these materials," she says. "Having a clear understanding of how these nanomaterials will change upon aging and consequent alterations in their physicochemical properties will enable establishing reliable structure-activity correlations. This is crucial in mitigating any unfavorable consequences of nanotechnology." | |
| As this work clearly shows, EHS researchers need to conduct studies on understanding the thermodynamics and the kinetics of the aging process for nanomaterials. Aging is strongly affected by environmental conditions. Additionally, as with all the properties of nanoscale materials, the thermodynamic and kinetic behavior will depend on their size and morphology. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
