| Posted: Apr 11, 2012 | |
Solar-powered cement production without carbon dioxide emissions |
|
| (Nanowerk Spotlight) The global cement industry is currently one of the largest single emitter of carbon dioxide, generating on average about 830 kg of this greenhouse gas for each 1000 kg of cement produced (source: International Energy Agency 2007, Tracking Industrial Energy Efficiency and CO2 Emissions). Considering that the worldwide annual production of cement is a whopping 3.8 trillion kg, the cement industry alone accounts for approximately 5-6% of man-made CO2 emissions. | |
| In a previous Nanowerk Spotlight ("New solar-powered process removes CO2 from the air and stores it as solid carbon") we introduced a novel solar conversion process, combining electronic and chemical pathways, for carbon dioxide capture. This STEP (Solar Thermal Electrochemical Production) process proactively converts anthropogenic carbon dioxide generated in burning fossil fuels, as well as eliminates carbon dioxide emissions associated with the generation of metals and bleach. In a subsequent Spotlight ("Reinventing iron production using clean renewable energy instead of coal") we showed how the STEP process could be used as an effective new carbon-dioxide-free process for iron production. | |
| The research team, led by Stuart Licht, a professor of chemistry at George Washington University, has now presented a solar-powered process to produce cement without any carbon dioxide. In a paper (accepted manuscript) in the April 5, 2012 online edition of Chemical Communications ("STEP Cement: Solar Thermal Electrochemical Production of CaO without CO2 emission"), they show that STEP-produced cement operates at solar energy conversion efficiencies higher than that in any solar photovoltaic. | |
 |
|
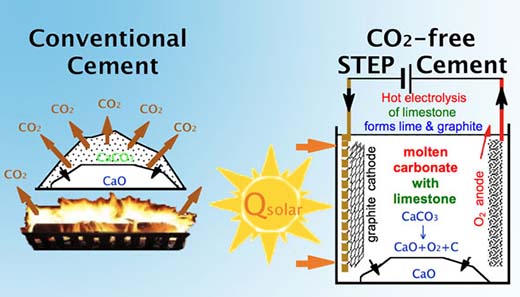
| Conventional thermal decomposition production of lime (left) versus STEP direct solar conversion of calcium carbonate to calcium oxide (right) eliminating CO2. (Image: Dr. Licht, University of Washington) | |
| "In cement production, the majority of CO2 emissions occurs during the decarbonation of limestone (CaCO3) to lime (CaO) and the remainder (30 to 40%) from burning fossil fuels, such as coal, to heat the kiln reactors to ∼900°C," Licht explains to Nanowerk. " Our study presents a low-energy, entirely new synthetic route to form CaO without any carbon dioxide emission, and is based on unexpected solubility behavior in molten salts. This synthesis can be accomplished without solar energy, and without our new STEP process, but is particularly attractive when combined with this new solar process." | |
| "Alternatively" he adds, "the new synthesis could be used by industry to produce cement using any non-solar, renewable or nuclear energy without any CO2 release, or greatly decrease CO2 if fossil fuels were used to drive the new cement production – in the latter, worst case scenario, the products are lime, graphite and oxygen; there is still no CO2 product, but CO2 would be used in the energy to drive the process." | |
| In STEP cement limestone undergoes low energy electrolysis to produce lime, O2 and reduced carbonate without carbon dioxide emission. | |
| "There have been proposals to form cement which recaptures or sequesters some of the CO2 emitted during its production process" says Licht. "This, however, is the first process which forms no CO2 while producing cement." | |
| In this new technique, the kiln limestone-to-lime process is replaced by an electrolysis process which changes the product of the reaction of the limestone as it is converted to lime. Rather than producing carbon dioxide, it reduces the carbon dioxide (adds electrons) and produces only oxygen and graphite (which can be readily stored as solid carbon) or CO for fuels, plastics or pharmaceuticals. This is accomplished at low energy and high throughput. | |
| The researchers plan to scale up the outdoor STEP cement prototype and in general want to increase the portfolio of useful chemicals made by their new solar process. | |
| "The goals are to replace today's fossil fuel economy with a renewable chemical economy," says Licht. "It works fine in the lab but scale-up is the challenge." | |
| He points out that, although the process is entirely new, the individual components –solar towers, 24/7 operation storing solar energy with molten salts – are already in place. | |
| The bottom line of these research results is that solar energy can be used to efficiently make products without carbon dioxide, and at solar energy efficiencies higher than in photovoltaics. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
