| Posted: Sep 07, 2007 | |
Toxicity of nanoparticles in aquatic environments |
|
| (Nanowerk Spotlight) The much heralded nanotechnology revolution is not happening with a big bang that completely turns our lives upside down, but rather in a creeping stealth mode where many ordinary everyday products, from cosmetics and textiles to electronic devices, sporting goods and car paint increasingly contain engineered nanoparticles. Sometimes, these nanoparticles are just a smaller version of the material already used in a product, for instance zinc oxide in sunscreen lotions, sometimes these particles are a new addition to a product, as for example fullerenes added to oil lubricants to improve their performance. This trend of increasing use of engineered nanoparticles in commercial products raises the question of what happens at the end-of-life stage of these products, when they get disposed or recycled. Is there is a risk of these nanoparticles being released into the environment? And if yes, is there a risk of these nanoparticles causing harm? This is an area of nanotechnology risk research that remains largely unexplored. In a groundbreaking study to determine the effects of nanoparticles on aquatic organisms, scientists at the University of Wisconsin’s Great Lakes Water Institute in Milwaukee have demonstrated that all nanoparticles are not created equal - at least when it comes to their effects on aquatic organisms. They have also discovered that existing attitudes toward the safety of titanium dioxide may be dangerous. | |
| With the proposed use of nanoparticles to aid in the cleanup of industrial and medical wastes and as technological sensors, the information uncovered by this study may help scientists determine which nanoparticles may be best suited for environmental work, such as bioremediation following an oil or chemical spill, and which nanoparticles should be strictly regulated in terms of aquatic exposures. | |
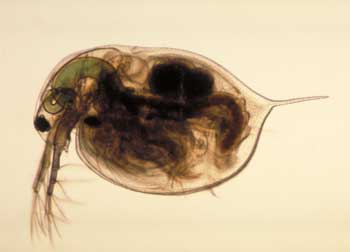
| The Wisconsin scientists – from the University of Wisconsin-Milwaukee and the Great Lakes WATER Institute – undertook the study to determine the potential risk of nanoparticles in a freshwater system. According to the study, which was published in Environmental Toxicology and Chemistry ("Behavioral and Physiological Changes in Daphnia magna when Exposed to Nanoparticle Suspensions (Titanium Dioxide, Nano-C60, and C60HxC70Hx)"), Daphnia magna, a planktonic crustacean found in a variety of freshwater systems from swamps to lakes and streams – and the most popular live food for aquarium fishes – was studied because the species filters a large amount of water per hour. This means they have significant interaction with their aquatic environment, causing them to face a higher risk of exposure to pollutants than other aquatic species. | |
 |
|
| Daphnia magna | |
| The scientists prepared and used two of the most commonly manufactured and most promising nanoparticles – fullerenes (C60) and titanium dioxide (TiO2) – which differ in chemical composition. Fullerenes are used in numerous applications including semiconductors and energy storage. TiO2 is being used to develop a variety of nanotechnologies from self-cleaning surfaces to water purification. | |
| In previous studies, both types of nanoparticles have shown some biological activity – both potentially useful and potentially detrimental to human health. Fullerenes can bind to lipids in the liver and brain, which may make them beneficial in drug delivery. But, this same binding characteristic, has also been shown to produce oxidative stress compounds in fish. Titanium dioxide inhibits the growth of cancer cells, but has shown other adverse effects in rodents. | |
| As the primary purpose of the study was to determine if certain nanoparticles would be better suited for environmental work than others, scientists exposed D. magna to varying concentrations of both nanoparticles in order to determine the differences and similarities of exposure between the two chemically different particles. Researchers hypothesized that because TiO2 is hydrophilic and C60 is hydrophobic, D. magna would react differently to exposure to each of the nanoparticles. They also believed that the smaller size of C60 (the average diameter of TiO2 is 10-20 nm, while the average diameter of C60 is only .72 nm) would cause the former to be more detrimental to the species. | |
| Researchers reported that exposure to filtered forms of both nanoparticles resulted in mortality for D. magna. However, it took far smaller concentrations of C60 than TiO2 to cause toxicity, which is in agreement with the researchers hypothesis that the smaller particles would, in fact, be more detrimental. According to the study, previous research has lead to the belief that TiO2 has no health risks. However, because this study indicates both fullerenes and titanium dioxide as detrimental to the survival of D. magna, researchers say the safety of TiO2 must be further evaluated. | |
| According to the study, however, exposure to concentrations of unfiltered, sonicated TiO2 powder which contained both micro- and nanoparticles did not cause the significant level of mortality seen in the filtered solution, even when concentrations were significantly increased. Researchers believe this is because the particles in the sonicated solution clump together to create larger particles. | |
| Although mortality among D. magna was the primary indicator of the effects of these nanoparticles on the species, other non-lethal effects were observed. When exposed to C60, juvenile D. magna experienced little mortality within the first hour, but most experienced immobility (which in a non-laboratory setting may result in death though predation.) After 24 hours, some died, some continued to experience immobility and others were swimming normally. After 48 hours, the survivors that had regained mobility seemed to revert to the initial immobile condition. Adult D. magna exposed to C60 exhibited signs of sporadic swimming and disorientation. None of these behavioral changes was observed in similar tests using TiO2. | |
| The study’s authors hope the results of this study lay a foundation for further research into the effects of other nanoparticles on organisms. They intend to conduct further research into the effects of nanoparticles on reproduction and other physiological effects. With the promise of nanotechnology, they say, it is important that a great deal more research is conducted in order to ensure the technology has the least negative impact on the environment. | |
| By Cathy Garber, Copyright Nanowerk LLC |
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
