| Posted: May 28, 2012 | |
Ultrasmall nanoparticles show promise as anti-cancer agents |
|
| (Nanowerk Spotlight) Researchers in China, lead by Xing-Jie Liang at the National Centre for Nanoscience and Nanotechnology in Beijing, showed that nanoparticles smaller than 10 nm in diameter accumulate more efficiently and penetrate more deeply in tumors relative to their larger counterparts. Their findings, published in the April 27, 2012 online issue of ACS Nano ("Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo"), have significant implications for the development of nanomaterials to diagnose and treat cancer. | |
| Nanomaterials are currently under intense development as part of the next generation of cancer therapies. Several formulations are already applied clinically, and dozens more are undergoing clinical trials. One of the key benefits of nanomaterials over conventional small molecule drugs is their ability to exploit the 'enhanced permeability and retention' (EPR) effect. Tumors grow in a rapid, uncontrolled way and lack properly-developed blood and lymphatic vessels. The blood vessels that do run through a tumor often contain pores ranging in size from 100 nm to over 1 µm. | |
| In contrast, healthy endothelial cells are typically connected by tight junctions that are approximately 2 nm wide. The abnormally large vascular pores in a tumor allow circulating nanomaterials to move from blood into the tumor bulk in a process known as 'extravasation'. In healthy tissues, nutrients and small molecules that extravasate are quickly carried away by the flow of the interstitial fluid surrounding cells, known as lymph. The inadequate lymphatic system in a tumor prevents normal lymph drainage, allowing extravasated nanomaterials to remain within a tumor for a prolonged period of time. | |
| Although the conditions that bring about the EPR effect can enhance the accumulation of a nanomaterial in a tumor, they can also prevent the nanomaterial from penetrating deep within the tumor bulk. Lymphatic drainage creates a convective flow of fluid from blood through the interstitial space. When this convective flow is interrupted, as it is in a tumor, the transport of a nanomaterial is limited to diffusion. | |
| Most nanomaterials that are currently approved for clinical use are approximately 100 nm in diameter. Their large size prevents them from diffusing effectively within the tumor matrix. Instead, they tend to remain near the vessel wall, and can reach only the first few layers of tumor cells. As a result, therapeutic nanomaterials are ineffective at eradicating the tumorigenic cells that lie relatively far from the blood vessels. Eliminating cells near the vessels may slow tumor growth in the short term, but it does not eliminate the source of the disease. Recurrences following such treatment are common. | |
 |
|
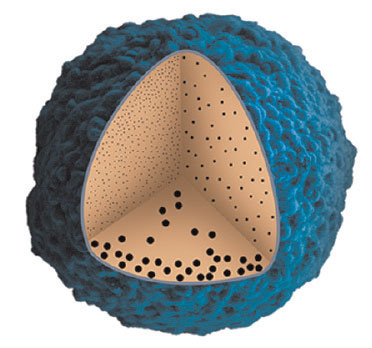
| Ultrasmall gold nanoparticles, less than 10nm in diameter, diffuse more deeply into tumors relative to their larger counterparts. (Reprinted with permission from American Chemical Society) | |
| One strategy to improve the diffusion of a nanomaterial within a tumor is to make it smaller. Several studies have shown that particles with diameters in the 10-30 nm range penetrate more deeply within tumors and enhance the therapeutic response. Until Liang's study, it was unclear whether 'ultrasmall' nanoparticles (those in the sub-10 nm range) would diffuse even deeper. Liang and his co-workers synthesized 2 nm, 6 nm, and 15 nm gold nanoparticles stabilized with the small thiolated molecule tiopronin. When they introduced these nanoparticles into a 3D breast cancer tumor model, known as a 'spheroid', they found that the 2nm particles accumulated over 10 times more efficiently and penetrated more deeply than the 15 nm particles. Similar results were observed when the nanoparticles were injected into mice bearing tumors derived from the same cell type. | |
| The enhanced tumor accumulation of the ultrasmall nanoparticles may be due, at least in part, to their prolonged blood circulation time. Most nanomaterials that enter the blood are rapidly cleared by tissue-resident macrophages in the liver and spleen. These cells are specialized to recognize foreign material directly or through adsorbed blood proteins known as 'opsonins'. While the majority of the 15 nm nanoparticles were cleared from the blood within minutes, the 2 nm nanoparticles remained in circulation for hours. The prolonged blood residence time of the small nanoparticles gives them more opportunity to extravasate into the tumor. | |
| The unique behavior of the ultrasmall nanoparticles extended to the sub-cellular level. As opposed to the 15 nm nanoparticles, which were trapped within vesicles in the cell cytoplasm, the 2 nm and 6 nm gold nanoparticles diffused throughout the cytoplasm and even entered the cell nucleus. This is significant since arresting the growth of cancer cells often requires access to its DNA. The cause of this unique sub-cellular distribution is not well-understood. A plausible explanation is that ultrasmall nanoparticles can diffuse directly across the lipid bilayer that forms the cell membrane, or pass through the tiny pores that are dotted within it. | |
| Ultimately, the ability of ultrasmall nanoparticles to diffuse deep within the tumor bulk may enable the design of nanoparticles that can carry therapeutic and diagnostic agents more efficiently into tumors. However, Liang's study is only a first step. Gold nanoparticles have little applicability in the clinic. In future, the gold cores must be replaced with more relevant formulations. Moreover, thorough investigation is needed to ensure ultrasmall nanoparticles do not pose unforeseen health hazards. Although it is still early, the unique behavior of ultrasmall nanoparticles is sure to have a significant influence on the future design of nanomaterials for cancer diagnosis and therapy. | |
| By Carl Walkey, Integrated Nanotechnology & Biomedical Sciences Laboratory, University of Toronto, Canada. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
