| Posted: Jun 15, 2012 | |
Optical nanomanipulation kills or fuses individual cancer cells |
|
| (Nanowerk Spotlight) Researchers in Israel have found a unique way to affect cancer cells in a rather controllable manner. For example, they can kill these cells in various ways, or they can make them fuse together, as they like. They do so by using functionalized, 20 nm in diameter gold nanospheres and specific, intense laser pulses with a visible wavelength tuned to the plasmonic resonance of those particles. This technique may have an impact on various technologies which require sophisticated cell manipulation for therapeutic and drug development applications. | |
| The team, led by Dr. Dvir Yelin, a Senior Lecturer in Biomedical Engineering at Technion- Israel Institute of Technology, has published their findings in the May 16, 2012 online edition of Small ("Optical Nanomanipulations of Malignant Cells: Controlled Cell Damage and Fusion"). | |
| "What is unique about our technique is that it is highly controllable and repeatable," Dr. Limor Minai, a researcher in Yelin's group and first author of the paper, tells Nanowerk. "The structural stability of the nanospheres even after the strongest laser illumination allows them to deliver the effect over and over again, until the desired result is obtained." | |
 |
|
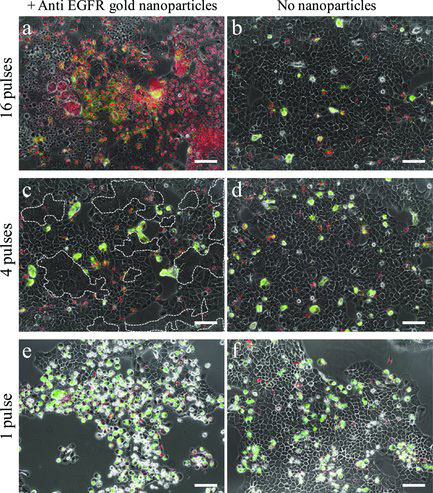
| Damaging carcinoma cells using anti-EGFR-coated nanoparticles and resonance femtosecond pulse irradiation. a) Gold-nanoparticle-conjugated cells irradiated by 16 pulses. b) Nonconjugated cells, 16 pulses. c) Conjugated cells irradiated by 4 pulses. d) Nonconjugated cells, 4 pulses. e) Conjugated cells irradiated by 1 pulse. f) Nonconjugated cells, 1 pulse. Multi-nucleated cells margins are marked by dashed white curves in (c). Scale bars represent 50 µm. Red nuclei indicate necrotic cells. Green stain indicates apoptosis. Panels a–d show cells 5 h after irradiation. Panels e,f show cells 23 h after irradiation. (Reprinted with permission from Wiley-VCH Verlag) | |
| Other similar approaches for nano-manipulations often suffer from particle melting and are therefore less effective in controlling the required process. | |
| The Technion researchers have developed this technique because they have realized that modern cancer treatments must become less intrusive, with minimal side effects and low damage to healthy tissue. They wanted an effective tool for manipulating cancer cells on a local, nanometric scale with minimal toxicity. | |
| As Minai explains, all three components of this novel technique – the gold nanoparticles, their targeting molecules, and the laser pulses – are considered non-toxic, but their combined effect is significant, and well controlled. | |
| "Perhaps the most important finding of this work is the ability to fuse together two or more neighboring cells," she says. "This finding could be important for numerous applications including cancer drug development and personal medicine." | |
| The use of gold nanoparticles in combination with laser pulses in photothermal cancer therapies has been demonstrated as an effective tool in previous research (see for instance our previous Nanowerk Spotlight: "Plasmonic nanobubbles combine diagnosis and treatment in one theranostic method"). Such approaches all follow three main steps: conjugating gold nanoparticles to specific molecules with high affinity to the targeted cells; delivering these nanoparticles to/into the cells; and irradiating them with intense laser pulses whose wavelength is tuned to the plasmonic resonance of the nanoparticles. | |
| This new work by Yelin's group follows the same approach for cancer cell nanomanipulations, but uses femtosecond pulses and gold nanospheres which are characterized by a much higher structural stability compared to other, nonspherical nanoparticles. | |
| One problem that could be addressed right away using this technique is the fusion of two specific cells out of a large heterogeneous population of cells. Using current fusion techniques specificity is difficult to obtain. This ability is achieved owing to the specific targeting of cells of choice by the nanoparticles, and illumination of the entire collection of cells with the laser beam. | |
| "Specific cell fusion would be of interest for drug development applications, where specific cell hybrids are needed to be formed, producing monoclonal antibodies which are known as effective anti-cancer drugs," explains Minai. "Another potential application involves the production of autologous cancer vaccines which is the result of fusion between the patient's cancer cells and his own immune system cells. The fusion product could then be re-injected to the patient for stimulating his immune system to fight against the cancerous cells." | |
 |
|
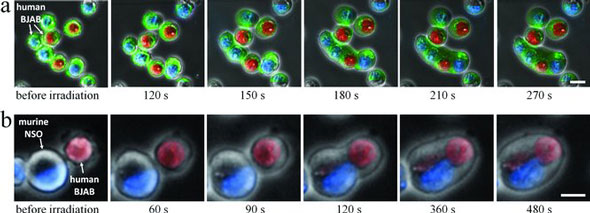
| Induction of cell fusion. a) Time sequence of fluorescence images of fusing B (BJAB) cells superimposed on phase contrast images, following irradiation by 5 pulses in the presence of nonspecific gold nanoparticles. Plasma membranes were labeled green. Nuclei were labeled either blue or red. b) Formation of a hybridoma cell. Time sequence of fluorescence images of human B (BJAB) cells (red nuclei) and murine myeloma (NSO) cells (blue nuclei) superimposed on phase contrast images, following irradiation by 5 pulses in the presence of nonspecific gold nanoparticles. Scale bars represent 10 µm. (Reprinted with permission from Wiley-VCH Verlag) | |
| The team is now trying to improve their technique to damage and fuse cancer cells and use it to develop therapeutic applications which would be tested on animals. | |
| "We are also trying to better understand the biological mechanisms behind the phenomenon we observed, which we currently don't fully understand," says Minai. "One challenge that we are facing is the relatively poor penetration depth of our laser pulses into deep tissue, particularly when compared to similar approaches which use near infrared light. Right now we address only applications which do not require deep penetration, and in parallel we develop techniques to overcome this issue." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
