| Posted: Aug 01, 2012 | |
Nanoparticles allow simple monitoring of cancer cells circulating in blood |
|
| (Nanowerk Spotlight) Early and accurate detection of cancer is critical for successful cancer therapies. In most cases, a tissue biopsy is the initial means of making a diagnosis. With increasing accuracy, "liquid biopsies" – where circulating tumor cells (CTCs) are isolated from blood samples – are becoming a viable complement or even alternative to invasive biopsies of metastatic tumors. CTC is of great interest for evaluating cancer dissemination, predicting patient prognosis, and also for the evaluation of therapeutic treatments, representing a reliable potential alternative to invasive biopsies and subsequent proteomic and functional genetic analysis. Unfortunately, given that human blood is a complex fluid that contains a variety of cells and metabolites, the fast detection of CTCs is quite a difficult task. | |
| "The main techniques reported for CTC detection consist in their labeling with tagged antibodies (immunocytometry) followed by fluorescence analysis or the detection of the expression of tumor markers by reverse-transcriptase polymerase chain reaction," Dr. Alfredo de la Escosura Muñiz, a senior researcher in the Nanobioelectronics & Biosensors Group at the Institut Català de Nanotecnologia, explains to Nanowerk. "However, the previously reported isolation of CTCs from human fluids are limited to complex analytic approaches that often result in a low yield and purity." | |
| In new work, Escosura and his collaborators, led by Arben Merkoçi, describe a rapid and simple electrochemical biosensing strategy to quantify circulating tumour cells based on the simultaneous use of antibody-coated magnetic beads, which selectively bind to the cancer cells for subsequent magnetic isolation, and antibody-coated gold nanoparticles, to also selectively bind to the cancer cells for final electrochemical detection. | |
| "We combine for the first time the use of both micro- and nanoconjugates and the simple electrochemical detection methodology for the sensitive and selective quantification of circulating tumour cells," says Escosura. | |
| The team has reported their findings in the July 20, 2012 online edition of Nano Letters ("Simple Monitoring of Cancer Cells Using Nanoparticles"). | |
 |
|
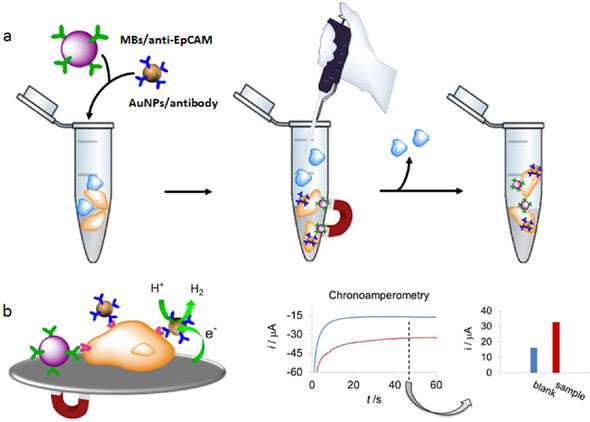
| Overall scheme. (a) Caco2 cells capture by MBs-anti-EpCAM and simultaneous labeling with AuNPs/specific antibodies in the presence of control cells. (b) Left: Detection of labeled Caco2 cells through the hydrogen evolution reaction (HER) electrocatalyzed by the AuNP labels. Right: Chronoamperograms registered in 1 M HCl, during the HER applying a constant voltage of -1.0 V, for AuNP labeled CaCo2 cells (3.5 × 104, red curve) and for the blank (PBS/BSA, blue curve). The comparison of the corresponding analytical signals (absolute value of the current registered at 50 seconds) is also shown. (Reprinted with permission from American Chemical Society) | |
| EpCAM (Epithelial Cell Adhesion Molecule) and CEA (Carcinoembryonic Antigen) proteins, both expressed by Caco2 cells, were selected as targets for the cells detection using biofunctionalized electrochemical labels. For the preparation of these labels, the team synthesized 20nm gold nanoparticles and then conjugated them with anti-EpCAM or anti-CEA antibodies. | |
| For the magnetic capture of Caco2 cells, EpCAM glycoprotein was used as a target for microbead capture, and mouse monoclonal anti-EpCAM was chosen to create biofunctionalized microbeads (uniform 2.8 µm polystyrene beads with a magnetic core) for the capture of CTCs. | |
| Escosura points out that the main advantages of the presented method, compared with the already reported ones for circulating tumour cells detection (i.e., optical methods, ELISA, RT-CPR), rely on the sensitive and quantitative electrochemical detection technique used. | |
| "The sensitivity, simplicity, low cost, easy-to-use mode, and miniaturization/portability of the electrochemical detection system makes it ideal for point-of-care applications," he says. "Furthermore, the use of gold nanoparticle electrocatalytic labels gives rise to very simple electrochemical records that allow the rapid quantification of the cells in the sample." | |
| The team envision the application of their method to the quantification of circulating tumour cells in real human samples where besides cells (cancerous and noncancerous ones) also proteins and metabolites are present. This method can also be adapted for other cancer cells by redesigning both micro- and nanoconjugates with the appropriate antibodies. | |
| Future research could take advantage of this technique for isolation, labeling and sensitive electrochemical detection/quantification of circulating tumour cells by incorporating it into lab-on-a-chip systems, which could contribute to the desired standardization of circulating cancer cells detection technologies. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
