| Posted: Sep 19, 2007 | |
Nanotechnology based magnetic separation could revolutionize separation technology |
|
| (Nanowerk Spotlight) Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry. | |
| Since magnetic separation is rapid, clean and proceeds at ambient conditions, why is it not used everywhere? In principle, it could, since biochemists have been using magnetic microbeads for over 20 years. What has limited the application of magnetic separation in chemistry is the fact that current materials' capacity is typically in the range of micromoles per gram – that means, one would require tons of magnetic agent if handling chemicals on a mole scale. In biochemistry, that works well, since proteins are used in a nano- to picomolar scale, but elsewhere, it makes little sense. | |
| This gap has now been bridged by researchers at ETH Zürich within the Functional Materials Laboratory (FML). | |
| "We used graphene sheets to cover metallic cobalt nanoparticles and chemically functionalized the graphene wrapping, thus linking organic chemistry and magnetic separation" Dr. Robert Grass explains to Nanowerk. "The use of 20-50 nm metallic cobalt particles allowed us to use one of the strongest magnetic materials in a safe-to-handle form. Normally, such small metal powders are pyrophoric and ignite spontaneously. We were able to show that the mere 1 nm carbon coating renders the particles stable in air or dilute acids. " | |
 |
|
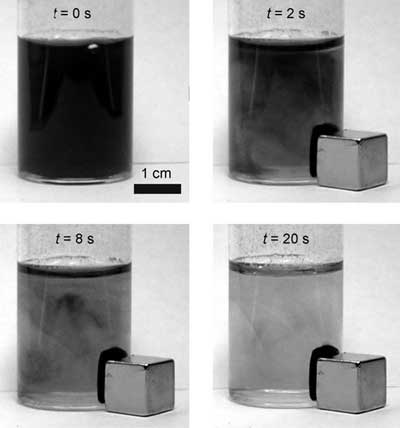
| Separation of cobalt nanoparticles from a suspension (1g per liter) in water by a commercial neodymium magnet (B=1.4 T). Photographs were taken at indicated times after placement of the magnet. (Reprinted with permission from Wiley) | |
| Grass, a postdoc researcher at Prof. Wendelin Stark's FML group at ETH Zürich, is the first author of a recent paper in Angewandte Chemie where the Swiss scientists describe their findings ("Covalently Functionalized Cobalt Nanoparticles as a Platform for Magnetic Separations in Organic Synthesis"). | |
| In their paper the researchers present the one-step, large-scale (>30g per hour) production of carbon-coated nanomagnets with high air and thermal stabilities. They demonstrate covalent functionalization of the carbon surface with chloro, nitro, and amino groups, the most frequently used linkers presently applied in solid supports. | |
| "We prepared the particles by reducing flame synthesis, a process which we recently derived from the industrially most prominent nanomaterials-manufacturing method, flame-aerosol synthesis; currently this method accounts for the preparation of several million tons of carbon, silica, and titania" says Grass. "We have most recently demonstrated the synthesis of pure cobalt nanoparticles ("Gas phase synthesis of fcc-cobalt nanoparticles"). These uncoated particles were only protected by oxide layers and could not be used as magnetic beads with a covalent functionalization." | |
| The core? shell arrangement presented in their recent paper was achieved by adding acetylene to the cobalt-nanoparticle-forming process, resulting in the controlled deposition of carbon on the particles. | |
| "This technology could revolutionize separation technology" says Grass. "I strongly believe that our process will soon work at a kilogram to ton scale. What we have to do now is to scale up the production of the beads." | |
| To that end, the Swiss researchers have turned entrepreneurs and founded an independent company called Turbobeads, based in Zürich, to facilitate the transfer of their invention from the research laboratory to the real world. ETH Zürich has an active program called "spinoff ETH Zürich" that supports technology transfer activities and start-ups based on ETH research. | |
| Turbobeads already offers their first product, amine-coated Turbobeads – carbon coated metallic cobalt nanoparticles, for sale via an online-shop. The company also has a number of functionalization chemistries in the pipeline. | |
| "With our current approach, we want to offer researchers throughout the world this new and fascinating separation tool" says Grass. "We feel that everybody should benefit from easier and time saving separation." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
